A sample of Ar(g) is contained in a
cylinder with a moveable piston at an initial
temperature of T. The volume of the
sample is decreased from 4.5 L to 1.5 L
while the pressure is held constant, as
shown in the diagram below.

Answers
Answer:
Option (B) is correct.
Explanation:
Initial temperature of the gas is T.
The volume of the sample is decreased from 4.5 L to 1.5 L while the pressure is held constant.
At constant pressure, the relation between volume and temperature is given by :
\(\dfrac{V_1}{T_1}=\dfrac{V_2}{T_2}\)
Here, V₁ = 4.5 L, V₂ = 1.5 L, T₁ = T₁, T₂ = ?
So,
\(T_2=\dfrac{V_2T_1}{V_1}\\\\T_2=\dfrac{1.5\times T_1}{4.5}\\\\T_2=\dfrac{T_1}{3}\)
So, the final temperature of the gas is \(\dfrac{T_1}{3}\). Hence, the correct option is (B).
Related Questions
The electronegativities of the period-3 elements are listed on the transparency. Calculate the
electronegativity differences for the following pairs of bonded period-3 atoms.
d. Si and Cl _______________________
b. Mg and S_1.31. And 2.58=
e. Si and S ________________________
c. Al and P ______________________
Answers
Here is the electronegativity difference of bonded period 3 atoms.
What is electronegativity?Electronegativity, is the symbolized as χ, it is the tendency to an atom of to a given chemical element to be attract shared electrons and when forming of a chemical bond. An atom's is electronegativity is affected by the both its atomic number .and it is
The electronegativity for period -3 atoms
Si and cl is - Electronegativity Difference is 0.7
Polarity-δ+Si−δ−C
For mg electronegativity difference is 1.3
For si and s electronegativity difference is 1.7
For AI and p the electronegativity difference is 1.6 and 2.9 respectively.
To know more about electronegativity click-
https://brainly.com/question/24977425
#SPJ1
A balloon originally had a volume of 4.39 l at 44 °c and a pressure of 729 torr. the balloon must be cooled to ________ °c to reduce its volume to 3.96 l (at constant pressure).
Answers
The balloon must be cooled to 39.69°C to reduce its volume to 3.96 l (at constant pressure).
According to Charles's law when the amount of gas and pressure are kept constant, the quotient that exist between the volume and the temperature will always have the same value.
\(\frac{V}{T}=k\)
Comparing an initial state 1 and a final state, it satisfied
\(\frac{V_{1} }{T_{1} } =\frac{V_{2} }{T_{2} }\)
Given information:
Initial volume: \(V_{1} =4.39l\)
Initial temperature: \(T_{1}=44\) °C
Final volume: \(V_{2} =3.96l\)
Final temperature: \(T_{2}=?\)
By applying Charles's law, we can find at which temperature the balloon must cooled to reduce its volume to 3.96L.
\(\frac{V_{1} }{T_{1} }=\frac{V_{2} }{T_{2} }\)
\(\frac{4.39}{44}=\frac{3.96}{T_{2} }\)
\(T_{2}=39.69\)°С
Hence, the balloon must cool to 39.69°C to reduce its volume to 3.96L.
Learn more about Charles's law visit:
brainly.com/question/3491421
#SPJ4
The balloon must be cooled to 39.69°C to reduce its volume to 3.96 l (at constant pressure).
According to Charles's law when the amount of gas and pressure are kept constant, the quotient that exist between the volume and the temperature will always have the same value.
\(\frac{V}{T}=K\)
Comparing an initial state 1 and a final state, it satisfied
\(\frac{V_{1} }{T_{1} } =\frac{V_{2} }{T_{2} }\)
Given information:
Initial volume: \(V_{1} =4.38l\)
Initial temperature: \(T_{1} =44\)°C
Final volume: \(V_{2}=3.96l\)
Final temperature: \(T_{2} =?\)
By applying Charles's law, we can find at which temperature the balloon must cooled to reduce its volume to 3.96L.
\(\frac{V_{1} }{t_{1} } =\frac{V_{2} }{T_{2} }\)
\(\frac{4.39}{44} =\frac{3.96}{T_{2} }\)
\(T_{2} =39.69\)°С
Hence, the balloon must cool to 39.69°C to reduce its volume to 3.96L.
Learn more about Charles's law visit:
brainly.com/question/3491421
#SPJ4
A chemical reaction produced 1.87 L of NO: gas at STP. How many moles of NO2 were produced in the reaction?
Answers
A chemical reaction produced 1.87 L of NO: gas at STP. 0.083 moles of NO2 were produced in the reaction.
To determine the number of moles of NO₂ produced in the reaction, you can use the ideal gas law at STP (standard temperature and pressure). At STP, 1 mole of any gas occupies 22.4 L.
The ideal gas equation is a formula that might be used to describe an ideal gas in its fictitious condition.Boyle's law, Charle's law, Avogadro's law, and Gay-Lussac's law are all combined in this formula. \(PV=nRT\) is the formula used, and the gas constant R has a value of 8.314.There are several restrictions in the legislation.Benoit Paul Emile Clapeyron introduced the statute in 1834.Ideal gases are subject to the ideal gas law.Ideal gas equation's graph is a straight line that goes through the origin.
Given that 1.87 L of NO₂ gas was produced, you can use the following proportion:
1.87 L (NO₂) / x moles (NO₂) = 22.4 L (any gas) / 1 mole (any gas)
To solve for x moles of NO₂:
x moles (NO₂) = 1.87 L (NO₂) × (1 mole (any gas) / 22.4 L (any gas))
x moles (NO₂) = 0.08348 moles
So, approximately 0.083 moles of NO₂ were produced in the reaction.
Learn more about ideal gas law here
https://brainly.com/question/20611809
#SPJ11
Once the following equation is balanced with the smallest set of whole number coefficients, what is the sum of the coefficients? (Don't forget to include coefficients of one.)Cr+H2SO4→Cr2(SO4)3+H2A. 11B. 4C. 13D. 15E. 9
Answers
The balanced equation for the reaction of chromium and sulfuric acid is: 2Cr + 3H2SO4 → Cr2(SO4)3 + 3H2. The sum of the coefficients is 9.
Make sure the number of atoms of each element on either side of the equation is equal. To do this, you can start by counting the atoms of each element on either side of the equation and making sure they are equal.
There are 2 chromium atoms on the left side, and 2 chromium atoms on the right side. There are also 3 hydrogen atoms on the left side, and 3 hydrogen atoms on the right side.
Finally, there are 3 sulfur atoms on the left side and 3 sulfur atoms on the right side.
Once you have established that the atoms are equal, you must then make sure that the coefficients are equal. To do this, you must multiply each atom on the left side by a coefficient.
The smallest set of whole number coefficients for this equation is 2Cr, 3H2SO4 on the left side, and Cr2(SO4)3 and 3H2 on the right side. This means that the sum of the coefficients is 9.
to know more coefficient refer here:
https://brainly.com/question/28975079#
#SPJ11
1.A space shuttle's wings are useless as it travels around the exosphere. Explain, in your own words, why the wings are so useless and how the shuttles can move around.
2.Explain, in your own words, the thermosphere and the stratosphere are our hottest atmosphere layers.
3.Explain, in your own words, how and why “shooting stars” occur. For example, why and what causes meteors to burn up.
4.Explain, in your own words, the importance of our ozone layer in the stratosphere.
5.Explain, in your own words, why weather only occurs in the troposphere.
PLEASE HELP!!!!! Use your own words please! I will give the brainiest to the person who answers it!
Answers
Thrust acts on the spacecraft and propels in the space.
How space shuttles move?1. The force created by the shuttle's engines in expelling the burning fuel produces an equal thrust in the opposite direction. This thrust acts on the spacecraft and propels in the space.
2. The thermosphere and the stratosphere are our hottest atmosphere layers because these two layers are present near the sun as compared to other layers of atmosphere.
3. When the meteors hit the atmosphere, meteors rub against air particles and create friction which is heating the meteors. The heat vaporizes most meteors, creating what we call shooting stars.
Learn more about atmosphere here: https://brainly.com/question/24925283
What’s the answer??????
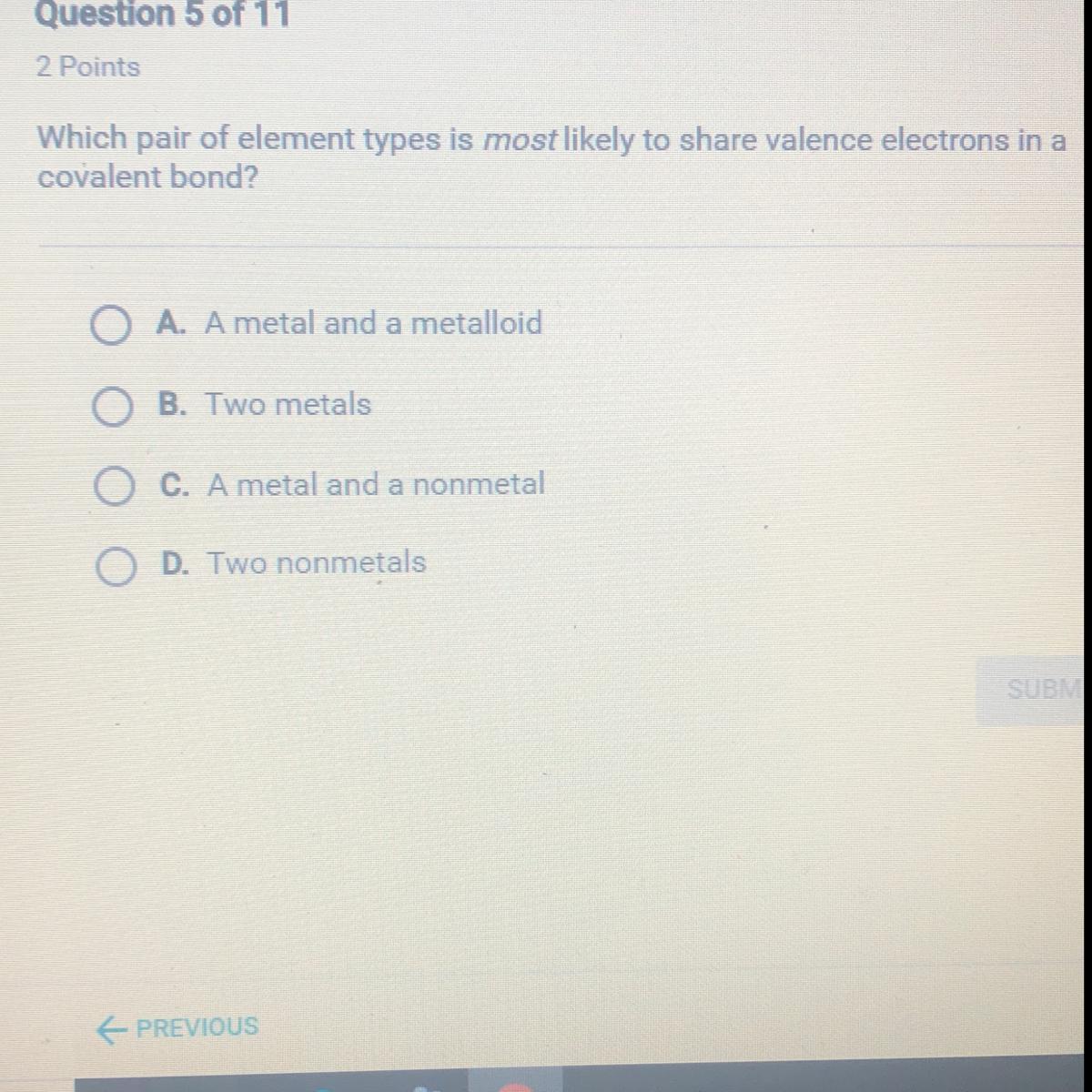
Answers
Answer:
D. Two non-metals
Explanation:
Non metals mostly form covalent bonds where they share electrons whereas, metal and nonmetal form ionic bonds where electrons are transferred.
What is occurring at the anode of the electrochemical cell containing the reaction represented by this equation?
Zn + Cu2+ → Cu + Zn2+
Answers
In the given chemical equation, oxidation takes place at anode of the electrochemical cell .
What is chemical equation?
Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ1
A published hypothesis:
A: does not need to be tested
B: becomes a scientific law
C: should be tested by the scientific community
D: is accepted by the scientific community
Answers
Answer:
C
Explanation:
I hypothesis needs to be tested to know whether it is true or not. Here is the definition:
A supposition or proposed explanation made on the basis of *limited evidence as a started point for further investigation*
Given the following Equation: _N2 + _H2 --> _NH3
How many grams of ammonia can be produced from 3.5 moles of nitrogen?
A)119g
B)247g
C)59.5g
D)45.5g
Answers
247g
HELP ME PLEASE!
What information in a balanced chemical equation shows how many moles of a reactant are involved in the reaction?
A. The superscripts in a formula tell the number of moles of the molecule.
B. The oxidation states of atoms in a molecule tell the number of moles.
C. The coefficient in front of the molecule tells its relative number of moles.
D. The subscript in a formula tells the number of moles of that molecule.
Answers
The information in a balanced chemical equation shows how many moles of a reactant are involved in the reaction is,
The coefficient in front of the molecule tells its relative number of moles.So, option C is correct one.
What is chemical equation?The symbolic representation of chemical reaction is called chemical equation.There are three parts of in chemical equation,first reactant from where reaction is started, second product the final result and arrows between reactant and product which shows conversion.The reactant part given in left hand side and product part is given in right hand side in chemical equation.What is balanced chemical equation?The chemical equation in which number of each type of atom is equal in both side of the arrow is called balanced chemical equation.
To learn more about chemical equation here.
https://brainly.com/question/12047033
#SPJ2
please help, i will give free kfc. i work in kfc.

Answers
Answer:Sexual reproduction can be described as the method of reproduction in which the offsprings produced will have half the chromosomes as compared to the parent cell. The other half of the chromosomes to make a complete set would arise from the other parent. In this way, the offspring produced will carry half of the chromosomes from the female parent and half from the male parent.
Crossing over and independent assortment are two phenomenons of meiosis due to which genetic diversity occurs and the offsprings born are not exactly similar to the parent cell.
Explanation:
what is organic chemistry
Answers
Answer:
Organic chemistry
Explanation:
Organic chemistry is part of the chemistry that studies carbon compounds, which also use organic compounds, which have characteristics.
Help me please!!!!!!!

Answers
Answer:
1. 264.369
2. 1772.65
3. 3.25
4.488
5. 0.164525
Explanation: I just added 0's to the ones that didnt have as many decimals which made it easy.
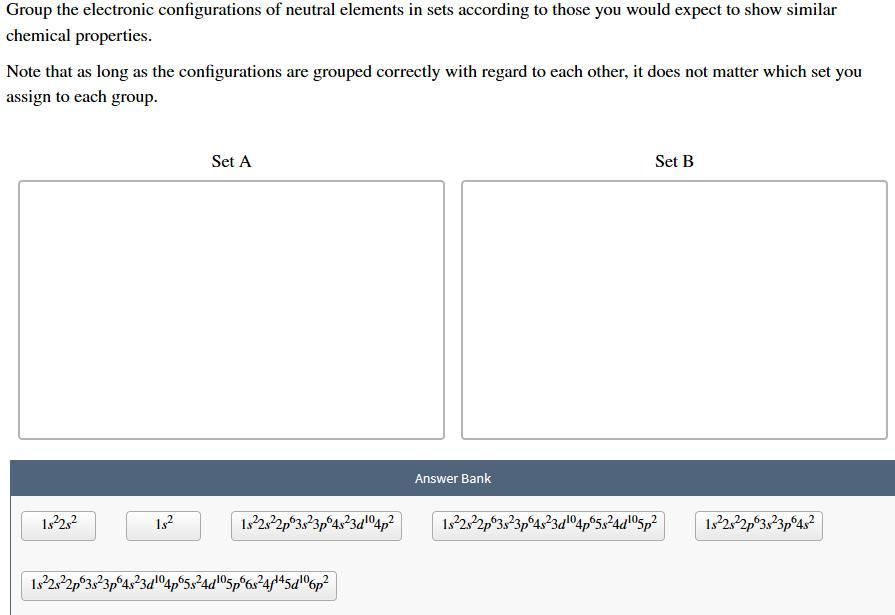
Group the electronic configurations of neutral elements in sets according to those you would expect to show similar chemical properties. Note that as long as the configurations are grouped correctly with regard to each other, it does not matter which set you assign to each group. Set A Set B Answer Bank 1:22:2 182 1922s22p03823p64323204p2 1322322p03823p 4323d"°4p65324d05p2 1922,22p3:23p6432 1.322s22p 3,23p64323 5p6s2445dº6p2
Answers
Set A elements behave similarly due to valence electrons in s and p orbitals, while Set B elements behave differently due to valence electrons in both s and d orbitals.
Set A:
1s²2s²
1s²
1s²2s²2p⁶3s²3p⁶4s²
Set B:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p²
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p²
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p²
The elements in Set A have valence electrons only in the s and p orbitals of their outermost energy level. This makes them similar in chemical behavior, as they tend to form covalent bonds by sharing electrons.
The elements in Set B have valence electrons in both the s and d orbitals of their outermost energy level, which gives them unique chemical properties, including the ability to form coordination complexes and exhibit variable oxidation states. Therefore, they are different in chemical behavior compared to the elements in Set A.
Learn more about neutral elements here: brainly.com/question/30433564
#SPJ4
Complete question is in the image attached below
what is M2 ?how does FOMCs could affect M2 and why ?
Answers
M2 refers to the money supply measure known as M2 money stock.
The FOMC's decisions and actions can impact M2 through its control over interest rates, open market operations, and the influence on economic conditions and confidence. Changes in monetary policy by the FOMC aim to manage the money supply and promote price stability, economic growth, and employment.
M2 is a broad measure of the money supply that includes cash, checking and savings deposits, money market funds, and other time deposits.
The Federal Open Market Committee (FOMC) is a committee within the U.S. Federal Reserve System that is responsible for making decisions regarding monetary policy, including setting interest rates and implementing measures to manage the money supply.
The actions taken by the FOMC can have an impact on M2 and the broader money supply in the economy. Here's how:
1. Open Market Operations: The FOMC conducts open market operations by buying or selling government securities in the open market. When the FOMC buys government securities, it injects money into the banking system, increasing bank reserves and potentially leading to an expansion of M2.
2. Interest Rate Policy: The FOMC sets the target federal funds rate, which is the interest rate at which depository institutions lend and borrow funds from each other overnight. By adjusting the federal funds rate, the FOMC influences borrowing costs for banks and, in turn, affects their lending practices. Changes in interest rates can impact the demand for loans and affect the growth of M2.
3. Impact on Confidence and Spending: The FOMC's actions and communications can influence consumer and business confidence. When the FOMC signals a more accommodative monetary policy stance, it can encourage borrowing and spending, potentially leading to an increase in the growth of M2.
To know more about Federal Open Market Committee (FOMC)
https://brainly.com/question/32317498
#SPJ11
A 353.2mL sample of chlorine gas is collected at 25.2°C and an atmospheric pressure of 100.8kPa What would the volume be at STP?
Answers
Answer:
Explanation:
To solve this problem, we can use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
At STP (standard temperature and pressure), the temperature is 0°C or 273.15 K, and the pressure is 1 atm or 101.325 kPa.
We can use the ideal gas law to find the number of moles of chlorine gas in the sample:
n = PV/RT
where P is the pressure, V is the volume, R is the gas constant, and T is the temperature in Kelvin.
First, we need to convert the given temperature of 25.2°C to Kelvin:
T = 25.2°C + 273.15 = 298.35 K
Now we can calculate the number of moles of chlorine gas in the sample:
n = (100.8 kPa)(353.2 mL)/(8.314 J/K/mol)(298.35 K)
n = 0.0158 mol
Next, we can use the number of moles and the ideal gas law to find the volume at STP:
V = nRT/P
V = (0.0158 mol)(8.314 J/K/mol)(273.15 K)/(101.325 kPa)
V = 0.364 L or 364 mL
Therefore, the volume of the chlorine gas at STP would be 364 mL.
which is the mostly likely atomic bonding type in a polyethylene polymer?
Answers
The most likely atomic bonding type in a polyethylene polymer is covalent bonding.Covalent bonds involve the sharing of electrons between atoms, resulting in strong bonds that hold the polymer chains together.
Polyethylene is a polymer composed of repeating units called monomers. In the case of polyethylene, the monomer is ethylene (C2H4), which consists of two carbon atoms and four hydrogen atoms.
The carbon atoms in ethylene form a covalent bond by sharing electrons. Each carbon atom has four valence electrons, and it shares one electron with the other carbon atom, forming a double bond. This double bond results in the carbon atoms being connected by a strong covalent bond.
The remaining valence electrons of carbon and hydrogen atoms form single covalent bonds to fulfill their electron requirements. Each carbon atom forms single covalent bonds with three hydrogen atoms, and the remaining valence electrons on the carbon atom form a bond with the other carbon atom.
Overall, the covalent bonding between carbon and hydrogen atoms in ethylene, and subsequently in the polyethylene polymer, is what holds the polymer chains together.
Based on the structure and bonding in ethylene and polyethylene, it can be concluded that the most likely atomic bonding type in a polyethylene polymer is covalent bonding. Covalent bonds involve the sharing of electrons between atoms, resulting in strong bonds that hold the polymer chains together.
To know more about vist:
https://brainly.com/question/3447218
#SPJ11
How do u study for a subject that you didn’t understand from the beginning of the year !!! (The subject is chem btw )
Answers
Answer: I would usually try my BEST to get help from yt videos. I recommend using yt that give the most kindergarten type speaking technique, this is really weird to say but it helps just incase you can be stressed out about the words being given by teachers in Chemistry. And I recommend writing down the notes when they explain as well. Hope this helps!
Ice sheets once covered the upper northeastern portion of the United States.
True or False?
Answers
Answer:
I do believe the answer is false.
Hope This Helps!!
What coefficient is needed to balance the reaction
CaCl2 + H₂CO3 → CaCO3 + HCI?
OA. 2CaCO3
OB. 2H₂CO3
OC. 2CaCl₂
OD. 2HCI
Answers
Answer:
OD. 2HCl
Explanation:
A balanced equation needs the equal # of each element on BOTH sides.
By putting a 2 in front of HCl you now have 2 hydrogens on both sides and 2 chlorines. The Ca and CO3 are already balanced.
What is the density of a substance with mass of 418.23g and a volume of 436.2ml
Answers
Answer:
0.96 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume}\\\)
From the question we have
mass = 418.23 g
volume = 436.2 ml
\(density = \frac{418.23}{436 .2} \\ = 0.958803\)
We have the final answer as
0.96 g/mLHope this helps you
Allison adds ice cubes to water which is at room temperature. Over time, the ice melts.
Read the statement above. How does adding ice to the room temperature liquid change the average kinetic energy of the particles of the
liquid?
Answers
Adding ice to the liquid decreases the average kinetic energy of the particles of the liquid.
The temperature of a substance is defined as a measure of the average kinetic energy of the molecules of the substance. The average kinetic energy of the molecules of a substance is directly proportional to the temperature of the substance.
This implies that if I increase the temperature of a substance, the average kinetic energy of the molecules of the substance increases and vice versa.
Ice is at a lower temperature than water at room temperature. Thus, adding ice to water at room temperature decreases the temperature of the water and consequently decreases the average kinetic energy of its particles.
Learn more: https://brainly.com/question/6670211
The number of waves that pass a given point in a certain amount of
time is the waves...
Answers
Answer:
100
Explanation:
nsvcscjkvschssvds
A 0.589 mol sample of nitrogen gas, N2, has a volume of 1.68L at a pressure of 0.975 atm. Calculate the temperature of the nitrogen gas
Answers
PV = nRT
Where:
P = pressure (in atm)
V = volume (in liters)
n = number of moles
R = ideal gas constant (0.0821 L·atm/(mol·K))
T = temperature (in Kelvin)
We are given:
n = 0.589 mol
V = 1.68 L
P = 0.975 atm
Rearranging the equation, we get:
T = PV / (nR)
Substituting the given values:
T = (0.975 atm) * (1.68 L) / (0.589 mol * 0.0821 L·atm/(mol·K))
Calculating the expression:
T ≈ 28.4 K
Therefore, the temperature of the nitrogen gas is approximately 28.4 Kelvin.
The temperature of the nitrogen gas is 33.92 Kelvin
How to solve:
To get the temperature of the nitrogen gas, we will use the ideal gas equation:
PV = nRT
where:
P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature. First, let's change the equation to account for temperature:
T = PV/(nR)
Presently, we can connect the given qualities:
The ideal gas constant, R, is 0.0821 L-atm/(mol-K), with P = 0.975 atm and V = 1.68 L and n = 0.589 mol.
Adding the following values to the equation:
T = (0.975 atm * 1.68 L) / (0.589 mol * 0.0821 L atm/(mol K)) T = 1.6332 atm L / (0.04813 L atm/(Kmol))
The nitrogen gas's temperature is 33.92 Kelvin because;
T = 1.6332 / 0.04813 K
T = 33.92 K.
Read more about Nitrogen gas here:
https://brainly.com/question/15022152
#SPJ1
A beaker contains a 25 ml solution of an unknown monoprotic acid that reacts in a 1:1 stochiometric ratio with naoh. Titrate the solution with naoh to determine the concentration of the acid.
Answers
The molarity of the acid is : 0.82 Molar
Calculate the molarity of acid?
Volume of acid = 25 ml
Molarity of base = 0.5 M
Volume of base = 41.00 ml
Since, acid and base reacts in 1:1 stoichiometric ratio, we can use the molar equivalence formula as:
M(acid) × V(acid) = M(Base) × V(Base)
M(acid) = 0.82 M
What is Molarity?
Molarity (M) is the amount of a substance in a given volume of solution. Molarity is defined as the number of moles of solute per liter of solution. Molarity is also called the molarity of a solution.
To know more about Molarity, check out:
https://brainly.com/question/26873446
#SPJ4
In three to five sentences describe the mistake the student made and determine whether or not the reaction is a redox reaction. explain your answer (4 points)
Answers
A redox reaction is when there is simultaneous oxidation and reduction. CuO+H2→Cu+H2O. Example: In this reaction, hydrogen is oxidised to water while copper oxide is reduced to copper.
By assuming that all bindings to the atoms in molecules are ionic, we can use the oxidation numbers assigned to atoms in molecules to detect redox reactions. In a reaction, oxidation is shown by an increase in the number of oxidations, and reduction is indicated by a copper. Consider the sodium-water reaction. In this reaction, sodium removes hydrogen from water while both becoming oxidised by obtaining oxygen and reducing the water to hydrogen by removing oxygen at the same time.
To learn more about oxidations, click here.
https://brainly.com/question/9496279
#SPJ4
Two different cars, the Model S and Model T, use different substances in their engines. The image above shows the two substances. At room temperature, both substances are liquids. A car mechanic transferred the same amount of energy out of the two containers, but only one substance changed phase. Which car’s substance changed phase, and how did it change?
Answers
Answer:
The Model T’s substance changed phase because the attraction of the molecules was able to overcome their slower movement. Its molecules now move in place.
Which activity is an example of a chemical change?
A) Dissolving table sugar in water.
B) Drops of water forming on a cup of iced tea on a hot day.
C) Melting gold to make jewelry.
D) An old penny rusting.
Answers
Answer:
D.an old penny rusting
Explanation:
A chemical property of iron is that it is capable of combining with oxygen to form iron oxide, the chemical name of rust.
An old penny rusting is an example of chemical change as it is accompanied by formation of a new substance.
What is a chemical change?Chemical changes are defined as changes which occur when a substance combines with another substance to form a new substance.Alternatively, when a substance breaks down or decomposes to give new substances it is also considered to be a chemical change.
There are several characteristics of chemical changes like change in color, change in state , change in odor and change in composition . During chemical change there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
There are three types of chemical changes:
1) inorganic changes
2)organic changes
3) biochemical changes
During chemical changes atoms are rearranged and changes are accompanied by an energy change as new substances are formed.
Learn more about chemical change,here:
https://brainly.com/question/23693316
#SPJ2
The enthalpy of combustion for octane (C8H18(l)), a key component of gasoline, is –5074 kJ/mol. The reaction equation is: C8H18(l) + 12.5O2(g) (arrow) 8CO2(g) + 9H2O(g).
What is the (triangle)H (subscript f) for this reaction?
16CO2(g) + 18H2O(g) (arrow) 2C8H18(l) + 25O2(g)
Answers
Answer:
10,148 kJ
Explanation:
Did it on Engenuity 2022 and got it right - just double 5074
Answer:
10,148
Explanation:
Find the heat produced from an 8.00 L cylinder of propane gas under 5.00 atm at 25.0 oC, if one mole of propane can produce 2220 kJ.
A. 4290 kJ
B. 0.0289 kJ
C. 877 kJ
D. 1.63 kJ
E. 5420 kJ
F. 1750 kJ
G. 8440 kJ
H. 1360 kJ
I. 37.2 kJ
J. 630 kJ
K. 266 kJ
L. 645 kJ
M. 2420 kJ
N. 7.36 x 10-4 kJ
Answers
Answer: 3597 kJ of heat
Explanation:
According to ideal gas equation:
\(PV=nRT\)
P = pressure of gas = 5.00 atm
V = Volume of gas = 8.00 L
n = number of moles = ?
R = gas constant =\(0.0821Latm/Kmol\)
T =temperature =\(25.0^0C=(25.0+273)K=298K\)
\(n=\frac{PV}{RT}\)
\(n=\frac{5.00atm\times 8.00L}{0.0821 L atm/K mol\times 298K}=1.63moles\)
As it is given :
1 mole of propane produces = 2220 kJ of heat
Thus 1.63 moles of propane produces = \(\frac{2200}{1}\times 1.63=3597kJ\)
Thus 3597 kJ of heat is produced