Answers
Density is a physical property of matter and is defined as the mass per unit volume of a substance. It can be calculated by dividing the mass of the substance by its volume.
To find the density of the metal, we can use the following formula:
Density = Mass/Volume
For the given sample, the mass is 20.11 g and the volume is 6.03 mL. So, we can plug in the values into the formula and get:
Density = 20.11 g / 6.03 mL
To get the final density of the metal, we need to convert the units of volume to cm^3 as the density is usually given in g/cm^3
1ml = 1cm³
So, 6.03 ml = 6.03 cm³
Density = 20.11 g / 6.03 cm³
Density = 3.344 g/cm³
Therefore the density of the metal is 3.344 g/cm³
Related Questions
An unknown mass of each of the following substances, initially at 23.0 ∘C , absorbs 1940 J of heat. The final temperature is recorded as indicated. Find the mass of each substance. Pyrex glass (final temp:55.4 C)
Answers
The mass of the Pyrex glass that absorbs 1940J of energy is 79.52grams.
How to calculate mass?The mass in a calorimetry procedure can be calculated using the following formula:
Q = mc∆T
Where;
Q = quantity of heat absorbed or releasedm = mass of substancec = specific heat capacity = 0.753Jg/°C∆T = change in temperatureAccording to this question, an unknown mass of each of the following substances, initially at 23.0∘C , absorbs 1940 J of heat. The final temperature is recorded as indicated. The mass can be calculated as follows:
1940 = m × 0.753 × (55.4°C - 23.0°C)
1940 = 24.39m
m = 79.52grams.
Therefore, 79.52grams is the mass of the pyrex glass.
Learn more about mass at: https://brainly.com/question/28992424
#SPJ1
In Thomas Cole's The oxbow who is the one figure depicted in the landscape and what is he doing
Answers
Answer:
In Thomas Cole's painting "The Oxbow," Thomas Cole, the artist, shows himself sitting in the landscape while painting it.
Explanation:
In the painting called "The Oxbow" by Thomas Cole, the artist actually put himself in the picture. He's sitting right in the middle of the landscape, just like he placed himself in the painting, as if taking a selfie while working on it. It's like he took a snapshot of himself while he was working on the painting. This shows how much he cared about creating the artwork and how he felt a personal connection to the beautiful scenery. It's really fascinating because it gives us a glimpse into how he saw himself as an artist and how he wanted to capture the incredible beauty of nature through his art.
How do molecules work?
Answers
A molecule is essentially created by a collection of atoms linked by a network of bonds.
they are called -----metals are good at transferring electric charge
Answers
Answer:
Copper Copper Copper Copper
HELP PLS Asap Why do slimmer objects go faster than things with more structure (use scientific terminology)
Answers
There are several factors that can contribute to the speed of an object, including its mass, shape, and the surface it is moving on. In general, slimmer objects tend to go faster than objects with more structure because they offer less resistance to movement, which allows them to accelerate more quickly and reach higher speeds.
One factor that can affect the speed of an object is its mass, or the amount of matter it contains. All other things being equal, an object with a lower mass will tend to be faster than an object with a higher mass, because it has less matter to move and therefore requires less energy to accelerate.
Another factor that can affect the speed of an object is its shape, or the way it is structured. Slimmer objects tend to be more streamlined, meaning they have a shape that allows them to move through the air or water with less resistance. This can allow them to go faster than more structured objects, which may have a shape that creates more drag or resistance to movement.
Finally, the surface an object is moving on can also affect its speed. For example, an object moving on a smooth, flat surface may be able to go faster than an object moving on a rough or uneven surface, because the smooth surface offers less resistance to movement.
Overall, the speed of an object is determined by a combination of these and other factors, including the force applied to the object and the level of resistance it encounters. Slimmer objects tend to go faster than objects with more structure because they offer less resistance to movement, which allows them to accelerate more quickly and reach higher speeds.
Name a liquid substance that could be used in the laboratory for: dissolving dry mortar on floor tiles; (i) removing KMnO, stains; drying acid anhydrides
Answers
A liquid substance that could be used in the laboratory for dissolving dry mortar on floor tiles is vinegar; (i) removing KMnO₄, stains is sodium metabisulfite solution; drying acid anhydrides is concentrated sulfuric acid.
What are solvents?Solvents are substances usually liquids, but may also be gases or solids that dissolve other substances known as solutes.
Solvents are usually used as cleansing agents.
One possible liquid substance that could be used for dissolving dry mortar on floor tiles is a mild acid solution, such as diluted hydrochloric acid or vinegar.
KMnO₄ stains are often difficult to remove, but one substance that can be used is sodium metabisulfite (Na₂S₂O₅) solution. Sodium metabisulfite acts as a reducing agent and can effectively neutralize and remove KMnO₄ stains.
Concentrated sulfuric acid is commonly used in the laboratory as a drying agent. It has a strong affinity for water and can efficiently absorb moisture, including water present in acid anhydrides.
Learn more about solvents at: https://brainly.com/question/25326161
#SPJ1
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins.
Dispersion Forces; Dipole-Dipole Forces; Hydrogen Bonding Forces
Kr, H2O, CHCI3, HF, C2H6, HBr
Answers
Answer:
Kr- Dispersion Forces
H2O- Hydrogen Bonding
CHCI3- Dipole-Dipole Forces
HF- Hydrogen Bonding
C2H6- Dispersion Forces
HBr- Hydrogen Bonding Forces
Explanation:
Dispersion forces occurs in all substances. They are the dominant intermolecular interaction in all non polar substances such as C2H6 and Kr.
Hydrogen bonding occurs when hydrogen is bonded to a highly electronegative atom such as Cl, Br, O etc. It is the dominant intermolecular interaction in HF, HBr and H2O.
Dipole-Dipole interactions occur when a permanent dipole exists in a molecule such as in CHCI3
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
All of the following ions have the ground-state electron configuration of a noble gas except which one? Select one: a. Ca2+ b. Cl- c. H- d. Al3+ e. Ga3+
Answers
Ga3+ do not have the ground state electron configuration of a noble gas.
electron configuration:
The arrangement of electrons in the atomic orbitals of an atom is called the electron configuration.
Noble gases have completely filled orbitals and belongs to group 18 which has outermost electronic configuration of ns2np6, where n is highest shell/period.
Ca2+ has an electronic configuration of [Ar]
Cl- has an electronic configuration of [Ar]
H- has an electronic configuration of [He]
Al3+ has an electronic configuration of [Ne]
Ga3+ has an electronic configuration of [Ar]d10
since, ga3+ has outermost electrons in its d subshell so, it do not have the ground state electron configuration of a noble gas.
learn more about electronic configuration:
https://brainly.com/question/29757010
#SPJ4
A gas occupying a volume of 656.0 mL at a pressure of 0.884 atm is allowed to expand at constant temperature until its pressure reaches 0.510 atm. What is its final volume?
Answers
Answer:
1.14 × 10³ mL
Explanation:
Step 1: Given data
Initial volume of the gas (V₁): 656.0 mLInitial pressure of the gas (P₁): 0.884 atmFinal volume of the gas (V₂): ?Final pressure of the gas (P₂): 0.510 atmStep 2: Calculate the final volume of the gas
If we assume ideal behavior, we can calculate the final volume of the gas using Boyle's law.
P₁ × V₁ = P₂ × V₂
V₂ = P₁ × V₁/P₂
V₂ = 0.884 atm × 656.0 mL/0.510 atm = 1.14 × 10³ mL
A ________________ causes a __________________ heat transfer rate.
Answers
Answer:
A smaller temperature difference causes a smaller heat transfer
Explanation:
The greater the temperature difference, the greater the rate at which heat transfers.
Nena___her hair every morning.
A.BRUSH
B.BRUSHES
C.BRUSED
D. WILLBRUSHED
Answers
Answer:
I'm pretty sure it's A. BRUSH
Explanation:
If I'm wrong let ne know please
Answer:
B.BRUSHES
Explanation:
a 135-g sample of a mental requires 2.50 kj to change its temperature from 19.5c to 100.0c what is the specific heat of this metal
Answers
Answer:
The specific heat of the metal is 0.223 J/(g·°C).
Explanation:
We can use the formula:
Q = m * c * ΔT
where Q is the amount of heat transferred, m is the mass of the metal, c is the specific heat of the metal, and ΔT is the change in temperature.
In this case, we know that the mass of the metal is 135 g, the change in temperature is ΔT = 100.0°C - 19.5°C = 80.5°C, and the amount of heat transferred is Q = 2.50 kJ = 2500 J.
Substituting these values into the formula and solving for c, we get:
c = Q / (m * ΔT)
c = 2500 J / (135 g * 80.5°C)
c = 0.223 J/(g·°C)
Therefore, the specific heat of the metal is 0.223 J/(g·°C).
Answer: 0.230 J/g°C
Explanation:
The equation of amount of heat gained/lost by a sample is defined as:
q = mCΔT
With q being the energy, m being the mass, C being the specific heat of the sample, and ΔT being the change in temperature. When solving for C, you get the equation:
C = \(\frac{q}{m(T_{f}-T_{i}) }\)
When you plug in your values, you get the answer:
C = \(\frac{2 500 J}{(135 g)(100.0-19.5) }\) = 0.230 J/g°C
What property can be easily measured in solids, liquids, and gases? (2 points)
Group of answer choices
The temperature of solids, liquids, and gases can be easily measured.
The texture of solids, liquids, and gases can be easily measured.
The color of solids, liquids, and gases can be easily observed.
The texture and temperature can be easily measured for solids, liquids, and gases.
Answers
Answer:
I think the answer is A
Explanation:
the temperature of solids , liquids and gases can be easily measured
Which one of the following is a balanced equation?

Answers
Hydrogen sulfide will burn \in three different ways, depending upon the amount of oxygen present. In one reaction, sulfur dioxide and water are produced. In the second reaction, water, sulfur dioxide, and sulfur are produced. The third reaction produces water and sulfur. What is maximum amount of sulfur dioxide that can be produced with 50.0g of hydrogen
Answers
The term mole concept is used here to determine the mass of sulfur dioxide.The mass of sulfur dioxide obtained from 50.0 g of hydrogen sulphide is 93.93 g.
One mole of a substance is defined as that quantity of it which contains as many entities as there are atoms exactly in 12 g of carbon - 12. The formula used to calculate the number of moles is:
Number of moles = Given mass / Molar mass
Here balanced reaction is:
2H₂S + 3O₂ → 2SO₂ + 2H₂O
n (H₂S) = n(SO₂)
n = 50.0 / 34.1 = 1.466
Molar mass of sulfur dioxide = 64.067 g/mol
Mass = n × M
m = 1.466 × 64.067 = 93.93 g
To know more about mole concept, visit;
https://brainly.com/question/19730733
#SPJ1
invenstigatory project potato battery research questions
Answers
Answer:
My hypothesis is that: If the electrolyte source is changed (potato, apple, lime, lemon), then the production of energy (measured in volts) using a lemon will produce the highest voltage because the acid content in the fruit or vegetable will produce electricity when in contact with the electrodes (both zinc and copper
Explanation:
What is the average density of the cobalt?
Answers
Answer:
8.86 grams per cubic centimeter
Density: 8.86 grams per cubic centimeter
Explanation:
Can someone help me with this pls

Answers
Answer:
i think the correct answer is A
Which activities can help conserve water when taking showers
Answers
Answer:
If you're ever shaving in the bathroom, turn the water off.
Explanation:
If you do this, you can save at least 3-4 pounds of water.
Answer:
The following activities can help conserve water while taking showers:
1) Lower shower time
2) Don't leave shower running.
3) Check for leaks
Explain how a rainbow is produced
Answers
A rainbow is produced through a proces that includes refraction, reflection, and dispersion of sunlight.
What more should you know about the production of rainbows?A rainbow is formed when sulinght is refracted and reflected by rain drops in the atmospher.
The sunlight is split into its component colors, which is why rainbows appear as having an array of colors. This is due to each color being bent by a different amount during refraction.
The colors of a rainbow are always in the same order, with red on the outside and violet on the inside.
Find more exercises on rainbows;
https://brainly.com/question/7965811
#SPJ1
Density of a substance D=M/V
Calculate the Density of the substance below.Explain by step by step.

Answers
The density of the substance is 200 kg/m³.
What is density?Density can be defined as the ratio of the mass and volume of an object.
The S.I unit of Density is kg/m³.
The formula of density of the substance is given as,
Formula:
D = m/V.......... Equation 1Where:
D = Density of the substancem = Mass of the substanceV = Volume of the susbtanceFrom the diagram,
m = 42 g = 0.042 kgV = lbh = 7×5×6 = 210 cm³ = 0.00021 m³Substitute these values into equation 1
D = 0.042/0.00021D = 200 kg/m³Hence, the density of the substance is 200 kg/m³.
Learn more about density here: https://brainly.com/question/1354972
#SPJ1
Answer:
It would be 200
Explanation:
Thank you
Fissure eruptions can produce a flattened layer of cooled lava called a lava ____.
Answers
Answer:
magma
Explanation:
brainly me and il give reason in comment
Substances A and B react with each other such that A is 75% consumed in 16 minutes and A is 87.5% consumed in 24 minutes. Changing the concentration of B has no effect on the reaction rate. The reaction is:
a. Zero order in both A and B.
b. First order in both A and B.
c. Second order in A and zero order in B.
d. First order in A and zero order in B.
e. There is insufficient information to answer this question.
Answers
Option d. First order in A and zero order in B. The reaction rate can be determined by the rate of change of concentration of the reactant over time.
In this case, the reaction is first order in A, meaning the reaction rate is proportional to the concentration of A. This can be seen as the reaction rate is the same even when the concentration of B is changed, implying that B has no effect on the reaction rate. On the other hand, the reaction is zero order in B, meaning the reaction rate is independent of the concentration of B. This means that the reaction rate is proportional to the concentration of A and is independent of the concentration of B. This can be observed from the fact that the reaction rate remains the same when the concentration of B is changed, implying that B has no effect on the reaction rate. On the other hand, the reaction rate is directly proportional to the concentration of A, meaning that as the concentration of A increases, the reaction rate also increases. Therefore, the reaction is first order in A and zero order in B.
Learn more about chemical reaction here: brainly.com/question/29762834
#SPJ4
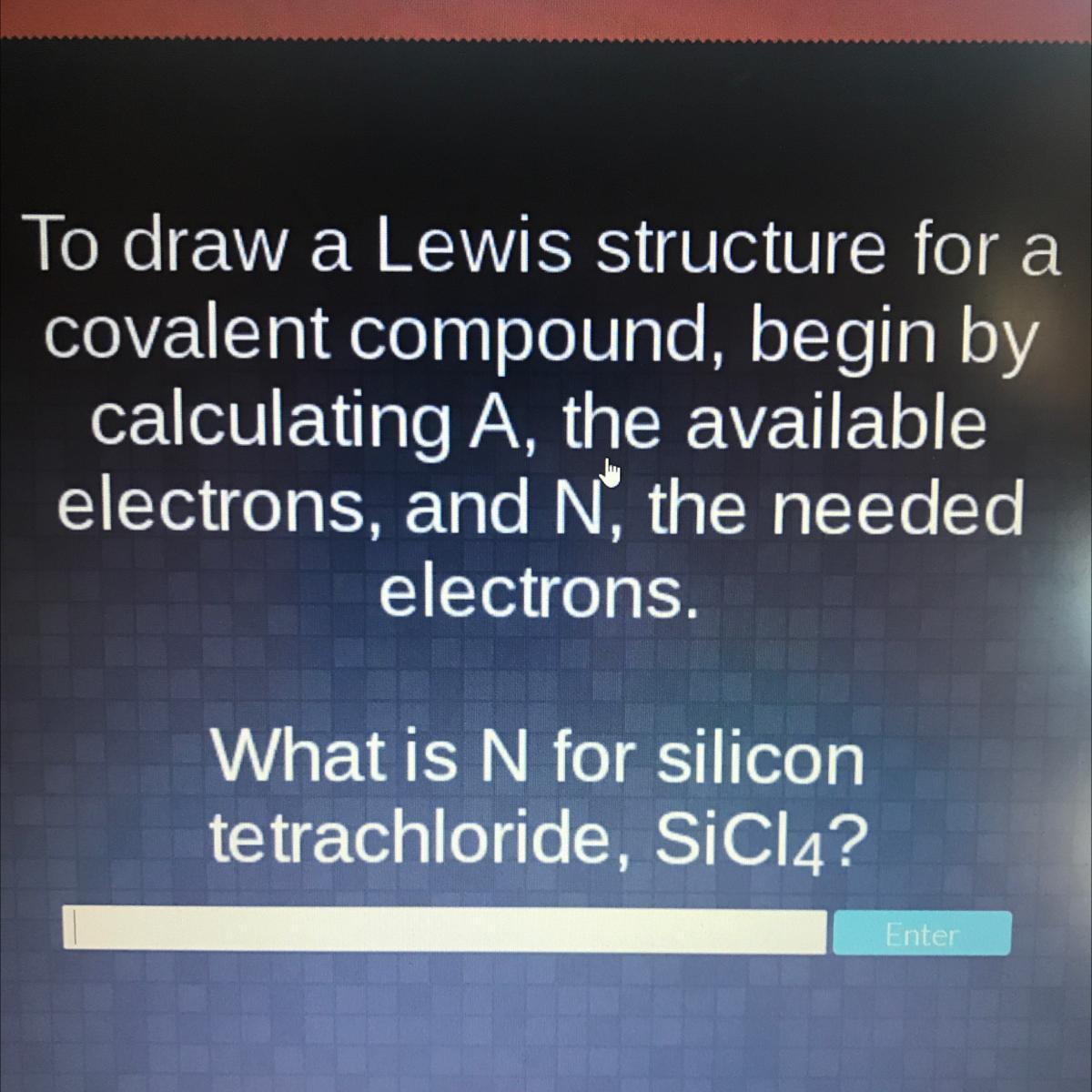
What is N for silicon tetrachloride, SiCl4?
Answers
A 1.50-L bulb containing Ne at 470 torr is connected by a valve to a 2.50-L bulb containing CF4 at 110 torr. The valve between the two bulbs is opened and the two gases mix. The initial gas pressures as known to three significant figures.
(a) What is the partial pressure (torr) of Ne?
(b) What is the partial pressure (torr) of CF4?
(c) What is the total pressure?
(d) What is the mole fraction of Ne?
Answers
(a) Partial pressure of Ne is 470 torr. (b) Partial pressure of of CF₄ is 110 torr. (c) Total pressure is 580 torr. (d) Mole fraction of Ne is 0.621.
(a) The initial pressure of Ne is 470 torr, so the partial pressure of Ne after mixing is also 470 torr.
(b) The initial pressure of CF₄ is 110 torr, so the partial pressure of CF₄ after mixing is also 110 torr.
(c) The total pressure is the sum of the partial pressures of the two gases:
Total pressure = partial pressure of Ne + partial pressure of CF₄
Total pressure = 470 torr + 110 torr
Total pressure = 580 torr
(d) To find the mole fraction of Ne, we need to know the number of moles of Ne and CF₄. We can use the ideal gas law to find the number of moles of each gas:
PV = nRT
n = PV/RT
For Ne:
n = (470 torr x 1.50 L)/(0.0821 L·atm/mol·K x 298 K)
n = 19.25 mol
For CF₄:
n = (110 torr x 2.50 L)/(0.0821 L·atm/mol·K x 298 K)
n = 11.72 mol
The total number of moles is:
nTotal = nNe + nCF₄
nTotal = 19.25 mol + 11.72 mol
nTotal = 30.97 mol
The mole fraction of Ne is:
XNe = nNe/nTotal
XNe = 19.25 mol/30.97 mol
XNe = 0.621
Therefore, the mole fraction of Ne is 0.621.
To know more about partial pressure please refer: https://brainly.com/question/16749630
#SPJ1
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
Given: H2 + O 2 → H2O1
the reaction occurs at ST.P a) Balance the chemical equation. (1 pts) b) Calculate the number of moles of the reactants needed to obtain 45 liner of H2O (2 pt) 4) Deduce the volume of the reactants (2 pts)
Answers
a) The balanced chemical equation for the reaction is: 2H₂ + O₂ → 2H₂O
b) the number of moles of O₂ required is approximately 1.004 moles.
c) approximately 45 liters of H₂ and 22.5 liters of O₂ are needed to obtain 45 liters of H₂O.
a) Balancing the chemical equation:
The balanced chemical equation for the reaction is: 2H₂ + O₂ → 2H₂O
b) Calculating the number of moles of the reactants needed to obtain 45 liters of H₂O:
From the balanced equation, we can see that for every 2 moles of H₂O produced, we need 2 moles of H₂ and 1 mole of O₂. Since the stoichiometry is based on moles, we need to convert the given volume of H2O into moles.
To convert volume to moles, we need to use the ideal gas law, PV = nRT. At standard temperature and pressure (STP), the molar volume of an ideal gas is 22.4 liters.
Given that we have 45 liters of H2O, we can calculate the number of moles as follows:
moles of H₂O = (volume of H₂O) / (molar volume at STP)
= 45 liters / 22.4 liters/mol
≈ 2.008 moles of H₂O
Since the stoichiometry of the reaction is 2 moles of H₂O for every 2 moles of H₂, we need an equal number of moles of H₂. Therefore, the number of moles of H₂ required is also approximately 2.008 moles.
For O₂, since the stoichiometry is 1 mole of O₂ for every 2 moles of H₂O, we need half the number of moles of H₂O. Thus, the number of moles of O₂required is approximately 1.004 moles.
c) the volume of the reactants:
Since the stoichiometry of the balanced equation is 2 moles of H₂for every 1 mole of O₂ and 2 moles of H₂O, we can deduce the volume of the reactants based on their molar volumes at STP.
For 2.008 moles of H₂, the volume can be calculated as follows:
volume of H₂= (moles of H₂) * (molar volume at STP)
= 2.008 moles * 22.4 liters/mol
≈ 45 liters of H₂
For 1.004 moles of O₂, the volume can be calculated similarly:
volume of O₂= (moles of O₂) * (molar volume at STP)
= 1.004 moles * 22.4 liters/mol
≈ 22.5 liters of O₂
Therefore, approximately 45 liters of H₂and 22.5 liters of O₂ are needed to obtain 45 liters of H₂O
for more questions on chemical
https://brainly.com/question/29886197
#SPJ8
Example Scenario:
Leah finished her lunch. All that is left is her plastic sandwich bag. Lea thinks there is no longer anything in the bag, but Paul disagrees. He thinks the bag is filled with air and air is something.
They decide to test their ideas by measuring and comparing the mass and volume of an empty flat and sealed plastic bag with one that has been inflated with air and sealed. Lea thinks that matter does not exist if it cannot be seen. Paul thinks matter can exist even when it's not visible.
Here is the data they collected:

Answers
The data collected by Leah and Paul supports Paul's opinion that matter can exist even when it is not visible.
What is support?Support is a term used to describe a range of services and resources offered to individuals in need. It can refer to providing help with practical tasks, such as helping someone to manage their finances or providing transport to medical appointments. It can also refer to providing emotional or social support. This could involve providing counselling or having regular conversations to help someone to feel understood and supported. Support can also refer to offering advice and guidance, or providing a safe space for someone to talk and express their feelings. In its broadest sense, support is about helping someone to have the best quality of life they can.
This is because the mass of the inflated bag is greater than the mass of the empty bag despite the fact that the volume of the inflated bag is much greater. This shows that the inflated bag contains air, which is matter, even though it cannot be seen.
To learn more about support
https://brainly.com/question/23849325
#SPJ1
4.A gas has an initial volume of 447 mL at 106 ºC and a final volume of 227 mL. What is its final temperature in degrees Celsius?
Answers
To solve this problem, we can use the combined gas law, which states that the ratio of initial pressure, volume, and temperature is equal to the ratio of final pressure, volume, and temperature. The formula is as follows:
(P₁ * V₁) / T₁ = (P₂ * V₂) / T₂
Given:
Initial volume (V₁) = 447 mL
Initial temperature (T₁) = 106 ºC
Final volume (V₂) = 227 mL
We need to find the final temperature (T₂). Let's assume the initial and final pressures are constant, so we can ignore them.
Now, we can plug in the values into the formula:
(447 mL * T₂) / (106 ºC) = (227 mL * T₁) / (106 ºC)
Simplifying the equation:
447 mL * T₂ = 227 mL * 106 ºC
T₂ = (227 mL * 106 ºC) / 447 mL
T₂ ≈ 54 ºC
Therefore, the final temperature of the gas is approximately 54 ºC.
Learn more about combined gas law, here:
https://brainly.com/question/30458409
#SPJ1