An alien civilization has different names and symbols for six elements. Place each element in the correct location in the periodic table.
Answers
Answer:
The picture
Explanation:
You just have to count the number of valence electrons and place the unknown elements on the periodic table according to their atomic number.

Answer:
Here they are.
Explanation:
I don't really know. Sorry. Hope This Helps, Though! :)

Related Questions
The chemical equation below shows the reaction between carbon dioxide (CO2) and lithium hydroxide (LiOH).
CO2 + 2LiOH -> Li2CO3 + H2OT
he molar mass CO2 is 44.01 g/mol. How many moles of LiOH are needed to react completely with 25.5 g of CO2?
a. 0.290
b. 0.579
c. 1.16
d. 1.73
Answers
1.16 moles of \(LiOH\) is required to react completely with 25.5 g of \(CO2\) where the molar mass \(CO2\) is 44.01 g/mol.
Given the reaction between carbon dioxide (\(CO2\)) and lithium hydroxide (\(LiOH\)) is as follows:
\(CO2 + 2LiOH - > Li2CO3 + H2O\)
The molar mass of \(CO2\) is given as: = 44g/mol
The mass of \(CO2\) is = 25.5g
Then the number of moles of \(CO2\) used is : = 25.5/44 = 0.579mol
The molar mass of \(LiOH\) is = 23.95 g/mol.
Since the equation states that 2 moles of \(LiOH\) are required to react with 1 mole of \(CO2\), then 2 x 0.579 mol = 1.16 mol of LiOH is required.
Hence 1.16 moles of \(LiOH\) is required to react completely with 25.5 g of \(CO2\).
To learn more about molar mass click here https://brainly.com/question/22997914
#SPJ4
what is the purpose of the methyl-red voges-proskauer (mrvp) test?
Answers
The purpose of the Methyl-Red Voges-Proskauer (MRVP) test is to differentiate between different groups of bacteria based on their metabolic pathways. This test consists of two components: the Methyl-Red (MR) test and the Voges-Proskauer (VP) test.
The Methyl-Red test is used to determine the ability of bacteria to perform mixed acid fermentation. Bacteria that produce large amounts of acid as byproducts of glucose metabolism will cause a pH drop in the medium, turning the methyl-red indicator red. This indicates a positive MR test. It is commonly used to identify bacteria belonging to the Enterobacteriaceae family, such as Escherichia coli.
The Voges-Proskauer test, on the other hand, detects the production of acetoin, a neutral end product of glucose metabolism. Bacteria that possess the enzymes necessary to convert glucose into acetoin will give a positive VP test. This test is often used to differentiate between members of the Enterobacteriaceae family, such as Klebsiella and Enterobacter species.
By performing the MRVP test, microbiologists can differentiate bacteria that follow different metabolic pathways. The results of this test provide valuable information about the biochemical characteristics of the bacterial strain being tested, aiding in their identification and classification.
Know more about Methyl-red voges-proskauer here:
https://brainly.com/question/31980982
#SPJ11
PLEASE HELPP!! What is the heat of reaction for the process NH4CI (s) ----> NH3 (g)+HCI (g)
Answers
Answer:
bblalabala;;a;;a
Explanation:
What is the heat of reaction for the process NH4CI (s) ----> NH3 (g)+HCI (g)
The reaction taking place is a gas-phase acid-base reaction (both HCl and NH3 are volatile), that produces particulate NH4Cl as the sole product: HCl(g) + NH3(g) → NH4Cl(s). 1.
If I add 26 mL of water to 172 mL of a 4 M NaOH solution, what will the molarity of the diluted solution be?

Answers
198 ml To create 4% NaOH, you will need 7.92 grammes of sodium hydroxide, or (4 x 198)/100. So, 7.92 grammes of sodium hydroxide are dissolved in 198 ml of water.
Does dilution lessen the molarity of a solution?The molarity of a solution is decreased by dilution. An aqueous solution's volume grows with the addition of more water, while the solute's moles remain constant. As a result, the solution's concentration and molarity are reduced.
What occurs as dilution increases?Dilution happens as the solution's volume increases. As just a result, conductivity decreases and ions per millilitre increase. The molar conductivity is calculated using one mole of ions. The molar conductivity of the solution rises as a result of increased ion separation and mobility.
To know more about hydroxide visit:
https://brainly.com/question/4251554
#SPJ1
In the reaction glucose + fructose → sucrose + water, __________ is a reactant and __________ is a product.
Answers
Answer:
glucose + fructose is a reactant, sucrose + water is a product
Explanation:
The first part of the equation is a reactant(s), and the other part of the equation is the product(s).
Give an example of a human activity that can alter chemical cycles and how.
Answers
Deforestation is an example of a human activity that can alter chemical cycles, specifically the carbon cycle. Deforestation refers to the permanent removal or clearing of forests or wooded areas, typically for human activities such as agriculture, logging, urban expansion, or the establishment of infrastructure.
When forests are cleared or destroyed through deforestation, the vegetation and organic matter in the forests are often burned or decomposed. This process releases a significant amount of carbon dioxide (CO2) into the atmosphere. Trees play a crucial role in the carbon cycle as they absorb CO2 through photosynthesis and store carbon in their biomass and soil. By removing forests, the natural carbon sink is diminished, and the balance of carbon dioxide in the atmosphere is disrupted.
Additionally, deforestation can lead to increased soil erosion. Without the tree roots and vegetation to hold the soil in place, erosion can occur more easily. This can result in the loss of valuable nutrients from the soil, such as nitrogen and phosphorus, which are essential for plant growth. The altered chemical composition of the soil affects nutrient cycling and availability for both plants and other organisms.
Overall, deforestation disrupts the natural balance of the carbon cycle and nutrient cycles, contributing to increased greenhouse gas emissions and nutrient depletion in ecosystems. It highlights how human activities can have significant impacts on chemical cycles and the functioning of ecosystems.
To know more about carbon cycle, click here, https://brainly.com/question/30508567
#SPJ11
Using immunoprecipitation, you can isolate a protein (protein X) you think is involved in chronic myelogenous leukemia (CML), which is caused, in part, by a hyperactive tyrosine protein kinase called ABL. You think one of the targets of ABL is the protein X that you can purify. In comparing tissues with and without the disease, you subject the samples to isoelectic focusing. Understanding how IEF works, how would you expect the samples with CML to migrate compared to the wild type, non-diseased sample protein?
A. The phosphorylated protein will have a lower isoelectric point and thus migrate further toward the anode.
B. The phosphorylated protein will be more attracted to the negative charge on the anode.
C. The non-phosphorylated proteins from the non-diseased samples will move further to the anode without the bulky phosphate group attached to a tyrosine.
D. Non-diseased proteins will migrate with a lower pl than those from diseased tissues and thus the samples from the non-diseased samples will migrate more to the anode than diseased protein.
Answers
The non-phosphorylated proteins from the non-diseased samples will move further to the anode without the bulky phosphate group attached to a tyrosine. The correct option is C.
Isoelectric focusing (IEF) is a technique used to separate proteins based on their isoelectric points (pI), which is the pH at which a protein has a net neutral charge. During IEF, proteins migrate in an electric field through a pH gradient until they reach a region of the gel where the pH matches their individual pI, resulting in immobilization.
In the context of chronic myelogenous leukemia (CML), where the hyperactive tyrosine protein kinase ABL is involved, the phosphorylation of proteins plays a significant role. Phosphorylation refers to the addition of a phosphate group to a protein. Phosphorylated proteins generally have a different charge distribution compared to non-phosphorylated proteins.
In this scenario, it is likely that protein X, which is suspected to be a target of the hyperactive ABL kinase, would be phosphorylated in the samples with CML. The addition of a phosphate group to the protein alters its charge distribution, leading to a change in its pI. Therefore, the phosphorylated protein X would have a different pI compared to the non-phosphorylated form.
Given this information, we can deduce that the non-phosphorylated proteins from the non-diseased samples would have a lower pI compared to the phosphorylated protein X from the CML samples. Consequently, the non-phosphorylated proteins would migrate further toward the anode in the isoelectric focusing process, as they would be attracted to the region of the gel with a higher positive charge.
This aligns with option C, which states that non-phosphorylated proteins from the non-diseased samples would move further to the anode without the bulky phosphate group attached to a tyrosine.
To know more about non-phosphorylated proteins refer here:
https://brainly.com/question/29472068#
#SPJ11
Match “the same” or “different” to each sentence.

Answers
Answer:
different , same, same, same
Need help to find the molarity. Please help!

Answers
Answer:
0.092M
Explanation:
Molarity of glucose solution = number of moles ÷ volume
According to this question, 12.5g of glucose (C6H12O6) is dissolved in enough water to make 750mL of solution.
Molar mass of glucose (C6H12O6) = 12(6) + 1(12) + 16(6)
= 72 + 12 + 96
= 180g/mol
mole = mass/molar mass
mole = 12.5/180
mole = 0.069mol
Volume of solution = 750mL = 750/1000 = 0.750L
Hence, molarity = n/V
Molarity = 0.069 ÷ 0.750
Molarity = 0.092M
why is carbon especially well suited to serve as the structural foundation of many biological molecules?
Answers
The significance of carbon bonding in biological molecules because it enables the synthesis of a variety of chemical compounds that are necessary for the survival and proper functioning of all living things.
Because it is the foundation for the structural and functional variety of biological molecules, carbon bonding plays a crucial role in these molecules. Carbon is a remarkable element that may be used to make a diverse range of chemical compounds because it can form stable covalent bonds with so many different elements, including other carbon atoms, hydrogen, oxygen, nitrogen, sulphur, and phosphorus. Carbon-based compounds make up the majority of biological molecules, including proteins, lipids, nucleic acids, and carbohydrates. These molecules are important for all living organisms to function and live.
Carbohydrates like sugars and starches are the primary energy source for all living things. Their structure is composed of covalently bonded simple carbohydrates like glucose and fructose. Fats and oils are lipids that are essential for the development of cell membranes as well as energy storage and insulation. Covalent bonds hold the fatty acids that make up their structure together. Nucleic acids like DNA and RNA make up the genetic material found in all living organisms. They are composed of nucleotides, which are connected by covalent bonds and are in charge of storing and transmitting genetic information. Proteins are essential for structure, regulation, and catalytic reactions in living things. The amino acids that makeup them are joined together by peptide bonds in their construction.
To know more about carbon bonding, click the below link
https://brainly.com/question/12156863
#SPJ4
What happens to a catalyst in a reaction?
Question 1 options:
a)
It is incorporated into the products.
b)
It remains unused.
c)
It is incorporated into the reactants.
d)
It evaporates.
Answers
Answer:
it's eveporates remains unused products
\(\huge{ \mathcal{ \underline{ Answer }\: \: ✓ }}\)
Catalyst are substance that are added to a reaction in order to speed of the reaction.
And they remain the same before and after the reaction, hence the correct option is :
B. It remains unused.
__________________________
\(\mathrm{ ☠ \: TeeNForeveR \: ☠}\)
is this virus real? i know it may not be but i wanna see your opinions.
Answers
Answer:
what
Explanation:
Which state of matter has a defined volume but no defined shape? 1 liquids 2 solids 3 gases 4 pizza
Answers
Answer:
the answer is liquids
Answer:
Liquids
Explanation:
Pizza is not a state of matter.
Liquids-a definite volume but no definite shape
Solids-a definite volume and shape
Gases-No definite volume or shape
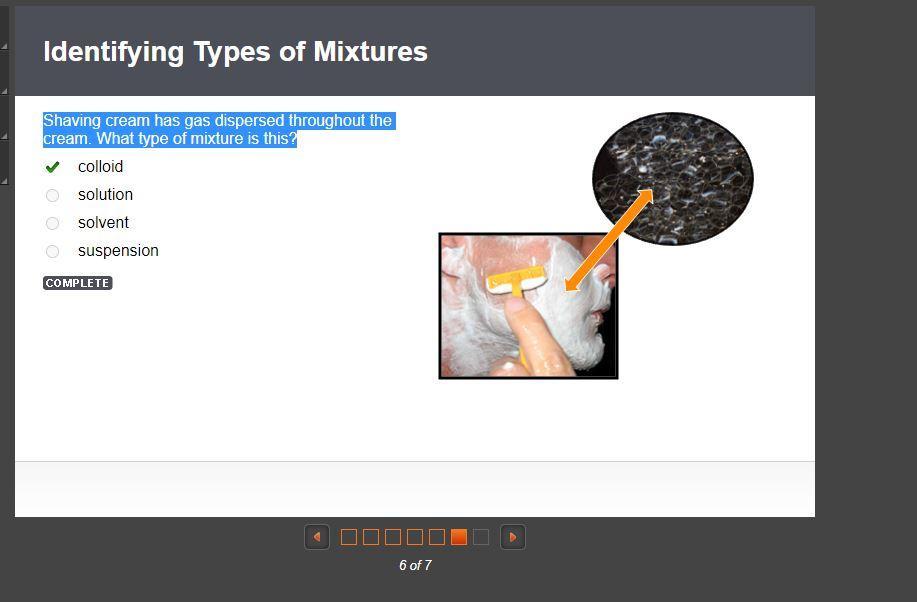
Shaving cream has gas dispersed throughout the cream. What type of mixture is this?
colloid is the answer
Answers
What was the name of the main scientist for the Manhatten Project
Answers
Answer:
Leo Szilard
Explanation:
Answer:
Robert Oppenheimer
Explanation:
Pls mark me brainless
how many moles of aluminum metal are required to produce 4.04 l of hydrogen gas at 1.11 atm and 27oc by reaction with hcl?
Answers
The moles of the aluminum metal are required to produce the 4.04 l of hydrogen gas at the 1.11 atm and the 27 °C by reaction with the HCl is 0.060 mol.
The ideal gas equation is as :
P V = n R T
Where,
P = pressure of the gas = 1.11 atm
V = volume of the gas = 4.04 L
T = temperature of the gas = 300 K
R = gas constant = 0.823 L atm K⁻¹ mol⁻¹
n = moles = P V / R T
n = ( 1.11 × 4.04 ) / ( 0.0823 × 300)
n = 0.181 mol of H₂
The chemical equation is :
2Al(s) + 6HCl(aq) ===> 2AlCl₃(aq) + 3H₂(g)
The moles of the Al = (2/6) 0.181 mol
The moles of the Al = 0.060 mol
To learn more about moles here
https://brainly.com/question/31597231
#SPJ4
How many grams of sodium iodide must be used to produce 44.8 grams of iodine in the following reaction? NaI+Cl2→I2+NaCl
Answers
26.4 grams of sodium iodide must be used to produce 44.8 grams of iodine in the given reaction : NaI+Cl₂→I₂+NaCl
The amount of sodium iodide (NaI) required to produce 44.8 grams of iodine (I₂) in the given reaction, we need to consider the balanced chemical equation and the molar masses of the substances involved.
The balanced equation shows that 1 mole of sodium iodide (NaI) reacts to produce 1 mole of iodine (I₂).
The molar mass of iodine (I₂) is 253.8 grams/mol.
Using these values, we can set up a proportion to calculate the amount of sodium iodide needed:
(44.8 g I₂) / (253.8 g/mol I₂) = (x g NaI) / (149.9 g/mol NaI)
Solving for x, the amount of sodium iodide needed, we have:
x = (44.8 g I₂) × (149.9 g/mol NaI) / (253.8 g/mol I₂)
x ≈ 26.4 grams
To learn more about sodium iodide refer here:
https://brainly.com/question/19952754#
#SPJ11
6. Although Inigo and the masked man are rivals, they actually have a lot
in common.
Give three examples from the swordfighting scene that show their
similarities.
Answers
The three (3) examples from the sword fighting scene that showed Inigo and the masked man's similarities are:
They can fight with both hands.They are both professional swordsmen. Inigo and the masked man discussed about their life.Who is Inigo?Inigo Montoya was one of the fictional character in the novel titled "The Princess Bride" and written by William Goldman's in 1973. Inigo hails from the village called Arabella and he was raised by his father Domingo Montoya, who was a master craftsman that makes sword.
In The Princess Bride, Inigo had a swordfight with a man in black (masked man) and they have the following things in common:
They can fight with both hands.They are both professional swordsmen. Inigo and the masked man spoke about their personal life.Read more on Inigo here: https://brainly.com/question/1130107
2. What is peat?
the aromatic compounds that are part of coal
O a soft, spongy material that may be changed into coal
O a mixture of methane, ethane, and other gaseous hydrocarbons
a very hard, dense form of coal
Answers
Answer:
a soft, spongy material that may be changed into coal
Explanation:
how many gr of MGSO4 do we get from the reaction of 2 moles MGO with H2SO4?
Answers
We can produce 240.74 g of Magnesium sulfate from the reaction of 2 moles of Magnesium oxide with Sulfuric acid.
How can you figure out how many grammes a reaction produces?As a result, we can see that we may obtain moles of our initial reactant by dividing its original mass in grammes by its molar mass. To obtain moles of product, multiply those grammes by the products-to-reactants ratio, which is 2:4. Lastly, we multiply the product's moles by its molar mass to obtain its grammes.
Magnesium oxide + Sulfuric acid → Magnesium sulfate + Water
The molar mass of Magnesium sulfate is:
Magnesium sulfate = 24.31 + 32.06 + (4 x 16.00) = 120.37 g/mol
So, 2 moles of Magnesium sulfate would have a mass of:
mass = 2 moles x 120.37 g/mol = 240.74 g
To know more about reaction visit:-
https://brainly.com/question/28984750
#SPJ1
The h ions produced by an acid in aqueous solution associate strongly with h2o to form the _____ ion, which has the formula _____.
Answers
The H ions produced by an acid in aqueous solution associate strongly with H₂O to form the Hydronium ion, which has the formula H₃O⁺.
Hydronium ion are formed when acid in introduced in an aqueous solution. It has the formula as H₃O⁺ formed from H₂O.
When Hydrogen ions associate with the water molecules formed from the acid solution, it forms Hydronium ions upon protonation.
Water molecule changes to Hydronium ions by accepting a proton.
Hydronium ions often used synonymously with Hydrogen ions.
Hydronium ions acts as an acid or base depending upon the Bronsted-Lowry concept.
Hydronium ions is a common name to the cation which is aqueous in nature.
It plays an important role while dealing with reactions that have a chemical nature occuring in aqueous solutions.
Hydronium ions is an important weathering agent.
Learn more about Hydronium ions here, https://brainly.com/question/14619642
#SPJ4
experiments also show that any aqueous solution at 25 degree celsius, the ionic-product of water Kw is equal to a constant value:
Answers
In any aqueous solution at 25°C, the ionic-product of water (Kw) is a constant value equal to 1.0 x 10⁻¹⁴ mol²/L².
Due to the auto-ionization or self-ionization of water, water molecules dissociate into hydronium ions (H₃O⁺) and hydroxide ions (OH⁻). At 25°C, the Kw is equal to 1.0 x 10⁻¹⁴ mol²/L².
This constant value of Kw plays a crucial role in understanding the acidity and basicity of aqueous solutions. It helps to establish the relationship between the concentrations of hydronium and hydroxide ions, as their product remains constant at a given temperature. The pH and pOH scales are derived from this relationship, providing a convenient method for measuring the acidity or basicity of a solution.
In summary, the ionic-product of water, Kw, remains constant at 1.0 x 10⁻¹⁴ mol²/L² for any aqueous solution at 25°C. This constant is a result of the auto-ionization of water and helps to understand the relationship between hydronium and hydroxide ions in the context of acidity and basicity.
Learn more about ionic-product of water here: https://brainly.com/question/31640554
#SPJ11
PLS FAST WILL GIVE BRAINLIEST!!! Rocky road ice cream is an example of a _____________________ mixture.
Answers
Answer:
Rocky road ice cream is an example of a __________Homogeneous___________ mixture.
About which other carbon–carbon bonds may rotation occur on 2 methylhexane
Check all that apply.
C-1−C-2 bond
C-2−C-3 bond
C-4−C-5 bond
C-5−C-6 bond
C-2−C-7 bond
Answers
The carbon-carbon bonds where rotation can occur on 2-methylhexane are the C-1−C-2 bond, C-2−C-3 bond, and C-5−C-6 bond.
Rotation is possible around single bonds, and in 2-methylhexane, these bonds are all single bonds. The C-1−C-2 bond, C-2−C-3 bond, C-4−C-5 bond, and C-5−C-6 bond are all single bonds, allowing for free rotation. On the other hand, the C-2−C-7 bond is not present in 2-methylhexane and therefore rotation cannot occur on that specific bond.
In 2-methylhexane, rotation can occur around the following carbon-carbon bonds:
C-1−C-2 bond: Yes, rotation can occur around this bond.
C-2−C-3 bond: Yes, rotation can occur around this bond.
C-4−C-5 bond: No, rotation cannot occur around this bond because it involves the methyl group attached to the second carbon, which creates a hindered rotation.
C-5−C-6 bond: Yes, rotation can occur around this bond.
C-2−C-7 bond: No, rotation cannot occur around this bond because it involves the methyl group attached to the second carbon, which creates a hindered rotation.
To learn more about 2-methylhexane
https://brainly.com/question/16945061
#SPJ11
Which observation is the best indication that a chemical reaction has occurred?A. Change in temperatureB. Presence of ashesC. Change from liquid to gasD. Absence of fizzing
Answers
Option (A). The best indication that shows a chemical reaction has occurred is the change in temperature.
A chemical reaction occurs when compounds or substances undergo a chemical change to form different compounds or substances. The substance initially involved in a chemical reaction are called reactants. Chemical reactions are usually characterized by a chemical change and they yield one or more products which usually have properties different from the reactants. When a chemical reaction occurs there is certain indication that shows the occurrence of chemical reaction. Those are,
- A change of color occurs during the reaction.
- A gas is produced during the reaction.
- A solid product called a precipitate is produced in the reaction.
- A visible transfer of energy occurs in the form of light as a result of
- Production of an odor
- Change of Temperature
To learn more about Indications of Chemical reaction please visit:
https://brainly.com/question/2390639
#SPJ4
What is the mass of an element with 3 protons and 4 neutrons? Also, how many neutrons are in the nucleus of an atom that has an atomic mass of 36 and atomic number of 25?
Answers
Choose the mixed number that is equivalent to $ 5.08.
5 8/100
5 80/100
80/100
8/100
Answers
Answer:
a. 5 8/100 is equivalent to $ 5.08.
Explanation:
i hope this helps :)
A solution has [OH-]=3.5*10^-6. Based on that, what must be true about this solution?
![A solution has [OH-]=3.5*10^-6. Based on that, what must be true about this solution?](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/YW5TyfgGVLOydEVPBNUxrU45MizSGS3C.jpeg)
Answers
Answer:
Option B. It is a basic solution.
Explanation:
To know which option is correct, let us calculate the pH of the solution.
First, we shall determine the pOH of the solution. This is illustrated below:
Concentration of Hydroxide ion, [OH¯] = 3.5×10¯⁶
pOH =.?
pOH = –Log [OH¯]
pOH = –Log 3.5×10¯⁶
pOH = 5.5
Finally, we shall determine the pH. This can be obtained as follow:
pOH = 5.5
pH =?
pH + pOH = 14
pH + 5.5 = 14
Collect like terms
pH = 14 – 5.5
pH = 8.5
The pH scale reads as follow:
0 to 6 => Acidic
7 => Neutral
8 to 14 => Basic
Comparing the pH of the solution (i.e 8.5) with the pH scale, we can conclude that the solution is basic because the pH of solution lies between 8 and 14.
determine the final volume of a gas at 89kPa and 120c if it occupies 1.75L at 675c and 1.45atm
Answers
The final volume of the gas at 89 KPa and 120 °C, given that it occupies 1.75 L at 675 °C and 1.45 atm, is 1.20 L
How do i determine the final volume of the gas?First, we shall list out the given parameters from the question:
Initial volume of gas (V₁) = 1.75 LInitial temperature of gas (T₁) = 675 °C = 675 + 273 = 948 KInitial pressure of gas (P₁) = 1.45 atmFinal pressure (P₂) = 89 KPa = 89 / 101.325 = 0.878 atmFinal temperature (T₂) = 120 °C = 120 + 273.15 = 393 KFinal volume of gas (V₂) = ?The final volume of the gas can be obtained by using the combined gas equation states as follow:
P₁V₁ / T₁ = P₂V₂ / T₂
(1.45 × 1.75) / 948 = (0.878 × V₂) / 393
Cross multiply
948 × 0.878 × V₂ = 1.45 × 1.75 × 393
Divide both side by (948 × 0.878)
V₂ = (1.45 × 1.75 × 393) / (948 × 0.878)
= 1.20 L
Thus, from the above calculation, we can conclude that the final volume of the gas is 1.20 L
Learn more about volume:
https://brainly.com/question/14560487
#SPJ1
How is a hypothesis tested ?
PLEASE PLEASE ANSWER ILL MARK AS BRAINLIESTT!!!!
Answers
Answer:
hypothesis testing is usually used to assess the plausibility of a hypothesis by using sample data
Explanation:
hope this helps :) (please mark brainliest!)