An amateur entomologist captures a particularly excellent ladybug specimen in a plastic jar. The internal volume of the jar is 0.5L, and the air within the jar is initially at 1 atın. The bug-lover is so excited by the catch that he squeezes the jar fervently in his sweaty palm, compressing it such that the final pressure within the jar is 1.25 atm. What is the final volume of the ladybug's prison?
Answers
The final volume of the ladybug's prison is approximately 0.4 liters.
To determine the final volume of the ladybug's prison, we can use Boyle's Law, which states that the pressure and volume of a gas are inversely proportional at constant temperature. The equation for Boyle's Law is:
P1 * V1 = P2 * V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume, respectively.
In this scenario, the initial volume (V1) is given as 0.5 L, and the initial pressure (P1) is 1 atm. The final pressure (P2) is 1.25 atm. We need to find the final volume (V2).
Plugging the given values into the equation, we have:
1 atm * 0.5 L = 1.25 atm * V2
Simplifying the equation, we find:
0.5 L = 1.25 atm * V2
Dividing both sides of the equation by 1.25 atm, we get:
0.5 L / 1.25 atm = V2
V2 ≈ 0.4 L
For such more questions on volume
https://brainly.com/question/31454001
#SPJ8
Related Questions
The density of Diamond is 3.51 g/cm3, and the density of platinum is 21.43 g/cm3. If equal masses of diamond and platinum are transferred to equal volumes of water in separated graduated cylinders, which graduated cylinder would have the greatest volume change
Answers
Answer:
Explanation:
Diamond has lesser density than platinum . So , if we take equal mass of both , the volume of mass of platinum will be far less .
The density of both diamond and platinum are more than water so both of them will be drowned in water completely . They will not float . On being drowned , platinum will displace lesser volume of water because of its less volume . So volume change in case of platinum mass will be far less . The volume change for diamond will be more because of its bigger size.
Consider a cobalt-silver voltaic cell that is constructed such that one half-cell consists of the cobalt, Co, electrode immersed in a Co(NO3)3 solution, and the other half-cell consists of the silver, Ag, electrode immersed in a AgNO3 solution. The two electrodes are connected by a copper wire. The Co electrode acts as the anode, and the Ag electrode acts as the cathode. To maintain electric neutrality, you add a KNO3 salt bridge separating the two half-cells. Use this information to solve Parts B, C, and D.
A. The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction.
Type the half-cell reaction that takes place at the anode for the cobalt-silver voltaic cell. Indicate the physical states using the abbreviation (s), (l), or (g) for solid, liquid, or gas, respectively. Use (aq) for an aqueous solution. Do not forget to add electrons in your reaction.
B. The half-cell is a chamber in the voltaic cell where one half-cell is the site of an oxidation reaction and the other half-cell is the site of a reduction reaction.
Type the half-cell reaction that takes place at the cathode for the cobalt-silver voltaic cell. Indicate physical states using the abbreviation (s), (l), or (g) for solid, liquid, or gas, respectively. Use (aq) for an aqueous solution. Do not forget to add electrons in your reaction.
Answers
Answer:
Anode half reaction;
Co(s) ----> Co^2+(aq) + 2e
Cathode half reaction;
2Ag^+(aq) + 2e-------> 2Ag(s)
Explanation:
A voltaic cell is an electrochemical cell that spontaneously produces electrical energy from chemical reactions. A voltaic cell comprises of an anode (where oxidation occurs) and a cathode (where reduction occurs). The both electrodes are connected with a wire . A salt bridge ensures charge neutrality in the anode and cathode compartments. Electrons flow from anode to cathode.
For the cell referred to in the question;
Anode half reaction;
Co(s) ----> Co^2+(aq) + 2e
Cathode half reaction;
2Ag^+(aq) + 2e-------> 2Ag(s)
In the reaction below what is the molar enthalpy if 1.73 mol A reacts with unlimited B and releases 4567 kJ of heat.
2 A+ 3 B - 2C

Answers
The standard enthalpy change for the reaction 2A+B⇌2C+2D is 664 kJ/mol and The heat that is absorbed when 3.70 mol of A reacts is 2456.8 J
The heat changes that take place as reactants combine to generate a product are measured by the enthalpy of a reaction.
The following formula can be used to determine the enthalpy change of a reaction:
Hess's law states that
Enthalpy of reaction = product's enthalpy - the reactant's enthalpy.
Considering the given reaction: 2A + B ⇌ 2C + 2D
Enthalpy of reaction = product's enthalpy - the reactant's enthalpy.
Enthalpy of reaction (ΔH°f) = (2 C + 2 D) - (2 * A + B)
Enthalpy of reaction (ΔH°f) = {[2(223) + 2(-523)] - [2(-245) + 2(-387)]}
Enthalpy of reaction (ΔH°f) = 664 kJ/mol
ΔH = q ÷ n
ΔH = molar enthalpy (heat) of solution
q = amount of energy (heat) released or absorbed
n = moles of solute
so. q = ΔH xn
q = ΔH xn
q = 664 kJ/mol x 3.70 mol
Q= 2456.8 J
Learn more about enthalpy of reaction at:
brainly.com/question/14291557
#SPJ1
all of these phytochemical compounds have been studied for their potential lipid-lowering effects except for _____. a.Sterols. b.Flavonoids. c.Anthocyanins. d.Mercury
Answers
Mercury is a heavy metal of considerable toxicity
Scientific literature reveals various plants and plant derived natural products, i.e., phytochemicals, which can alleviate experimentally induced mercury toxicity in animals.
Phytochemicals have great antioxidant potential and are of great interest due to their beneficial effects on health of human beings, and they give immense health benefits to the consumers.The effects of phytochemical-rich foods on bioaccessibility of mercury in fish tissue (the amount of mercury that is released from fish into gastrointestinal tract fluid following a simulated digestion) were investigated using an in vitro digestion.
To know more about Mercury :-
brainly.com/question/9418447
#SPJ4
Calculate how many grams of MgCl2 are needed to produce 12.15 grams
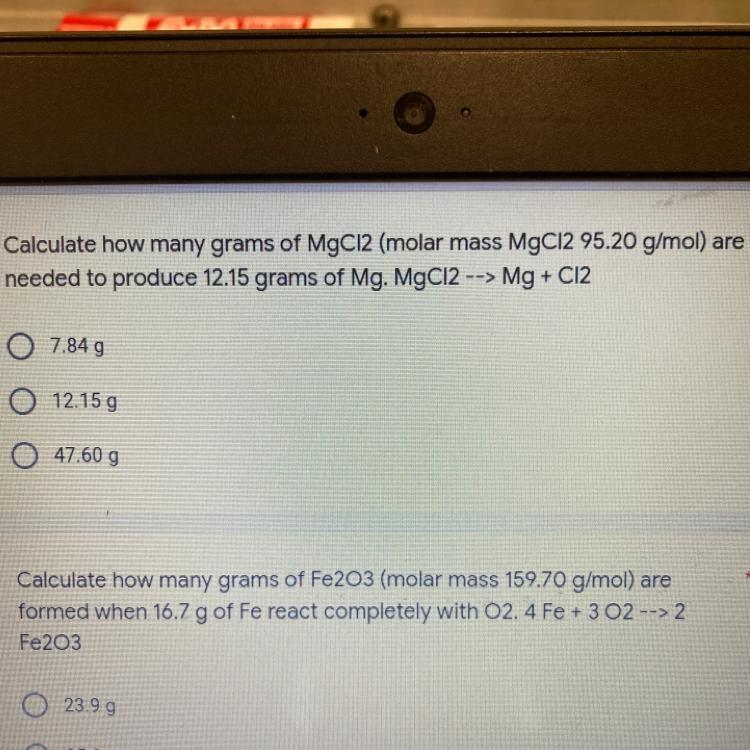
Answers
Answer
47.60 g
Explanation
Given that:
Molar mass of MgCl₂ = 95.20 g/mol
Mass of Mg produced = 12.15 grams
Equation: MgCl₂ → Mg + Cl₂
What to find:
To calculate the grams of MgCl₂ needed to produce 12.15 grams of Mg.
Step-by-step solution:
Step 1: Convert the mass of Mg produced to moles.
Using the molar mass of Mg (24.305 g/mol) and the mole formula, the moles of Mg produced is
\(Moles=\frac{mass}{molar\text{ }mass}=\frac{12.15\text{ }g}{24.305\text{ }g\text{/}mol}=0.49989714\text{ }mol\)Step 2: Determine the moles of MgCl₂ needed to produce 12.15 g of Mg.
According to the given equation; 1 mol of MgCl₂ produced 1 mol of Mg
So, x mol of MgCl₂ will produce 0.49989714 mol of Mg
That is:
\(\begin{gathered} 1mol\text{ }MgCl₂=1mol\text{ }Mg \\ \\ x=0.49989714mol\text{ }Mg \\ \\ x=\frac{0.49989714mol\text{ }Mg}{1mol\text{ }Mg}\times1mol\text{ }MgCl₂ \\ \\ x=0.49989714mol\text{ }MgCl₂ \end{gathered}\)Step 3: Convert the moles of MgCl₂ needed to produce 12.15 g of Mg to grams.
Using the molar mass of MgCl₂ = 95.20 g/mol, therefore the mass in grams of MgCl₂ needed to produce 12.15 g of Mg will be:
\(\begin{gathered} Mass\text{ }of\text{ }MgCl_2\text{ }needed=Molar\text{ }mass\times Moles \\ \\ Mass\text{ }of\text{ }MgCl_2\text{ }needed=95.20\text{ }g\text{/}mol\times0.49989714\text{ }mol \\ \\ Mass\text{ }of\text{ }MgCl_2\text{ }needed=47.60\text{ }g \end{gathered}\)Hence, the grams of MgCl₂ needed to produce 12.15 grams of Mg is 47.60 g.
4. The half-life of F-20 is 11.0 s. If a sample initially
contains 5.00 g of F-20, how much F-20 remains after
44.0 s
Answers
The amount of the F–20 that will remain after 44 s given the data from the question is 0.3125 g
Data obtained from the question Half-life (t½) = 11 sOriginal amount (N₀) = 5 gTime (t) = 44 sAmount remaining (N) = ?How to determine the number of half-lives Half-life (t½) = 11 sTime (t) = 44 sNumber of half-lives (n) =?
n = t / t½
n = 44 / 11
n = 4
How to determine the amount remaining Original amount (N₀) = 5 gNumber of half-lives (n) = 4Amount remaining (N) = ?N = N₀ / 2ⁿ
N = 5 / 2⁴
N = 5 / 16
N = 0.3125 g
Learn more about half life:
https://brainly.com/question/26374513
#SPJ1
count the number of atoms of each of the following elements and record them on the table down below

Answers
Answer:
Explanation:
Here, we want to count the number of atoms of each element
Let us start with the reactant side
There are 2 hydrogen atoms (symbol H)
There are 2 chlorine atoms (symbol Cl)
There are 2 Zinc atoms (symbol Zn)
For the product side:
There are 2 Zinc atoms (dark circle)
There are 2 Chlorine atoms (Symbol Cl)
There are 2 hydrogen atoms (symbol H)
We have the equation of the model as:
\(2\text{HCl + 2Zn }\rightarrow2ZnCl_{}_{}+H_2\)What is paper made of?
Answers
Paper used as a writing material is made of pulp (wood).
What is paper?Paper is a sheet material used for writing on or printing on (or as a non-waterproof container), usually made by draining cellulose fibres from a suspension in water.
Paper is made from cellulose found in trees, which are the main source of cellulose fibre (or woodpulp). Besides woodpulp, paper can be made from other materials such as cotton, flax, esparto, straw, hemp, manilla and jute.
Wood pulp is usually a softwood, used for pulping to make paper.
Learn more about pulp at: https://brainly.com/question/23590026
#SPJ1
Why did Rutherford choose alpha particles in his experiment?
Answers
b) Ammonia and sulfuric acid react according to the equation given below. How many millilitres of 0.110 M sulfuric acid are required to neutralize exactly 25.0 mL of 0.0840 M NH3 solution? 2 NH3(aq) + H₂SO4 (aq) → (NH4)2SO4(aq)
Answers
The amount of 0.110 M sulfuric acid are required to neutralize exactly 25.0 mL of 0.0840 M NH3 solution is 9.55mL.
A Neutralization Reaction: What Is It?A neutralisation reaction is a chemical process in which an acid and a base combine to produce salt and water as the end products. H+ ions and OH- ions combine to generate water during a neutralisation process.
2 NH3(aq) + H₂SO4 (aq) → (NH4)2SO4(aq)
moles of NH3 = (25ml x 1L/1000mL) x 0.084M
=> 2.1x 10^(-3) moles
The mole ratio of NH3 to H₂SO4 in the given reaction
=> moles of H₂SO4 = 2.1 x 10^(-3) moles of NH3 x 1 molesH₂SO4/2 moles
NH3
=> 1.05 x 10^(-3) moles
Volume = moles/molarity
=> 1.05 10^(-3) moles/0.110M
=> 9.55 x 10^(-3) L = 9.55mL
Learn more about neutralization reaction here:
brainly.com/question/28970253
#SPJ1
1. During an investigation, a student measured the amount of space various objects take up. Which property of matter was the student investigating?
volume
mass
weight
density
Answers
Calculate the heat energy of liquid mercury at 28900 °C is converted to solid mercury at its melting point.
Answers
The heat energy given off when liquid mercury at 28.9 °C is converted to solid mercury at its melting point is 284.2 J.
What is the heat energy given off when liquid mercury at 28.9 °C is converted to solid mercury at its melting point?The heat energy given off when liquid mercury at 28.9 °C is converted to solid mercury at its melting point is determined as follows:
Moles of Mercury:
14.0 g Hg x 1 mol Hg/201 g = 0.0697 moles
Heat released:
q = heat = m x C x ∆T
25ºC = 298.15K
25ºC = 298.15K
∆T = 234.32 K- 298.15 K
∆T = 63.83 K
q = (0.0697 mol)(28.0 J/molK)(63.83K) = 124.6 J
The heat released from liquid to solid:
q = m x ∆H fusion = (0.0697 mol)(2.29 kJ/mol)
q = 0.1596 kJ
q = 159.6 J
The total heat energy is given off:
Qtotal = 124.6 J + 159.6 J
Qtotal = 284.2 J
Learn more about heat energy at: https://brainly.com/question/934320
#SPJ1
Complete question:
Calculate the heat energy released when 14.0 g of liquid mercury at 28.9 °C °C is converted to solid mercury at its melting point.
Q. 2 A and B are powders, A is insoluble while B dissolves to give a pH 3 solution. Mixing A and B gives bubbles or effervescence and a clear solution. Which is the acid? If the other substance is a carbonate, name the gas given off. Even though A is insoluble a clear solution is given off, explain why.
Answers
Answer:
From the information provided, it seems likely that substance A is an acid, and substance B is a carbonate. The fact that mixing the two substances results in bubbles or effervescence, and that a clear solution is formed, suggests that a chemical reaction is taking place.
Acids and carbonates react together to form a salt, water, and carbon dioxide gas. The reaction is as follow:
Acid + Carbonate → Salt + Water + Carbon Dioxide
The carbon dioxide gas forms bubbles in the solution and causes effervescence, and the clear solution formed is due to the fact that both the acid and the carbonate have reacted together to form the salt and water. This reaction is acid-base neutralization reaction as the acid and base will neutralize each other.
As for the acid, since it is said that B gives pH 3 solution after dissolved, it can be inferred that it is not an acid, otherwise it would be acidic. Therefore A which is insoluble is the acid.
As for the gas given off, it's Carbon dioxide.
Explanation:
Gas Laws
Pre-Test Active
1
2 3
5
6
O final pressure
O atmospheric pressure
O combined pressure
O partial pressure
7 8
9
10
A scientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide,
oxygen, and nitrogen. Which term most likely describes what she is measuring?
Answers
The term that the scientist would use in this case is partial pressure. Option D
What is the partial pressure?
The pressure that one particular gas component within a mixture of gases exerts is referred to as partial pressure. It is the pressure that the gas would experience at the same temperature if it were the only thing in the entire volume.
When researching gas mixtures, such as in gas laws, gas phase equilibria, and gas collecting methods, partial pressures are extremely crucial for the gas.
Learn more about partial pressure:https://brainly.com/question/30114830
#SPJ1
Describe what type of electron transfer happens between these two atoms.

Answers
Answer:
Magnesium loses it's two electrons and Oxygen gains two electrons. Magnesium is now a cation and Oxygen is now an anion. Both become stable.
Explanation:
The type of electron transfer which happens between the Mg and O atom is a complete transfer of electrons from the Mg atom to the O atom in an ionic bond.
As we know, Ionic bonding is a type of chemical bonding characterized by the complete transfer of electrons from one atom to the other.The most electropositive electron usually is usually the donor while the most electronegative elements is the acceptor.
As evident in the case above;
Mg, which is the electropositive element with 2 Valence electrons loses its Valence electrons to become Mg²+.O, which is the electronegative element with 6 Valence electrons, and consequently, only needs 2 electrons to complete it's octet.Ultimately, an ionic bond is formed between the Mg and O atoms.
Read more:
https://brainly.com/question/21596753
water pollution ________ salt water levels in the sea.
A. rearranges
B. decreases
C. maintains
D. increases
Answers
Answer:
maintain
Explanation:
i don't know it's a guess
please helppp asaappppppp

Answers
Answer:
B. s, p, d, f
Explanation:
These things are often referred to as suborbitals and you normally have s,p,d,f.
S has 1 two orbitals
P has 3 orbitals
D has 5 orbitals
F has 7 orbitals
and each orbital can house 2 electrons
Which solution conducts electricity?
O Salt Solution
O Sugar Solution
O Both
O Neither
Answers
Explanation- This is because salt in an ionic compound and can easily move electrons between positive and negative ions.
which models show ions and which models show atoms?
Answers
Answer:
The correct statement is that both models represent atoms with the same atomic number.
Explanation:
Changes in the earth's climate can sometimes be measured in thousands of years (or more) false or true
Answers
Answer:
true
Explanation:
the climate can sometimes be measured in thousand
How many molecules are in 4.67 mols of H2O
Answers
Answer:
2.81 × 10²⁴ moleculesExplanation:
The number of molecules can be found by using the formula
N = n × L
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
We have
N = 4.67 × 6.02 × 10²³
We have the final answer as
2.81 × 10²⁴ moleculesHope this helps you
12 moles of Cu is how many grams of Cu?
Cu = 63.55 grams/mole
Answer needs to be rounded to 2 decimal places: 0.00

Answers
Answer:
762.60 g
Explanation:
In order to convert from moles of a substance into grams, we need to multiply the number of moles by the substance's molar mass:
Moles * Molar Mass = GramsWith the above information in mind, we can use the data given by the problem and calculate how many grams are there in 12 moles of Cu:
12 mol * 63.55 g/mol = 762.60 gThe melting point of ice is the same temperature as the ___ of water.
O Boiling point
O Condensation point
O Freezing point
Answers
Answer:
freezing point of water
Explanation:
I think it because the water change to ice
Answer:
freezing point
Explanation:
Question 5 of 10
Platinum has a density of 21 g/cm³. A platinum ring is placed in a graduated
cylinder that contains water. The water level rises from 4.0 mL to 4.2 mL
when the ring is added. What is the mass of the ring?
A. 2.6 g
OB. 3.8 g
OC. 4.2 g
OD. 5.2 g
B
Answers
Answer:
4.2 g
Explanation:
The VOLUME of the ring is 4.2 - 4.0 = .2 ml = .2 cm^3
the MASS of the ring is this times the density
.2 cm^3 * 21 g/cm^3 = 4.2 g
Answer:
the answer is c
Explanation:
density is mass/volume
so mass=density × volume
but we take the change is volume that is v2-v2=4.2-4=0.2ml
but the density is in gm/cm^3 so we should convert ml into cm^3. eventually they are equal so mass=21×0.2=4.2
what does rows represent on the periodic table
Answers
Answer:
Rows on the periodic table represents a period, which is the number of energy levels or rings. It is the last shell which valence electrons are on.
For example the first row waas Hydrogen and Helium which each have one energy level.
The esecond row has Carbon and Oxygen which has two energy level.
The third row has Sodium which has three energy levels and so on.
Help me please.
How do animals see their pray without light?
Answers
Answer:Many nocturnal animals have a mirror-like layer, called the tapetum, behind the retina, which helps them make the most of small amounts of light.
Explanation:
What does the 195 represent in the isotope notation?
195Pt
78

Answers
The representation that 195 has here is the atomic mass
What does the 195 represent in the isotope notation?
The number 195 in the isotope notation represents the mass number of the isotope. In this case, the isotope notation is for the element platinum (Pt) with a mass number of 195 and an atomic number of 78 (which is given as a subscript). The mass number is the sum of the number of protons and neutrons in the nucleus of an atom of the element. Therefore, the isotope notation for this element indicates that it has 78 protons and 117 neutrons (since 195 - 78 = 117).
Read more on atomic mass here:https://brainly.com/question/3187640
#SPJ1
If you just want points just say so this can lead me to failing 10th if I don’t pass this quiz so if you don’t know just say so I understand you want points I just don’t wanna fail Ocean acidification is a consequence that occurs when the equilibrium between CO2
CO
2
concentrations in the ocean and atmosphere starts shifting. What is the pH of pure water?
Question 8 options:
13, basic
7, neutral
4, acidic
Answers
Answer:
13,basic would be your answer!
Explanation:
PLS HELP!!!!
A system does 566 kJ of work and loses 216 kJ of heat to the surroundings.
What is the change in internal energy, Δ , of the system? Note that internal energy is symbolized as Δ in some sources.
Answers
Answer:
let a real person help u...
The change in internal energy of the system = -772kJ
Explanation:
Heat lost by the system , a = -266KJ
Workdone by the system, W = -506KJ
The first law of thermodynamics states that:
Change in internal energy = q + w
Substituting values into the equation
Change in internal energy = (-266KJ) + (-506KJ)
Change in internal energy = -722KJ
A gaseous mixture containing 7.00 moles of nitrogen, 2.50 moles of oxygen, and 0.500 mole of helium exerts a total pressure of 0.900 atm. What is the partial pressure of the nitrogen?
Answers
Answer:
Partial Pressure = 0.630atm
Explanation:
The total pressure of a mixture of gases is equal to the partial pressure of those gases. The partial pressure of a gas is defined as:
Partial pressure = Mole Fraction * Total pressure
The mole fraction of a gas is the ratio between the moles of the gas and the total moles.
To solve this question we need to find the mole fraction of nitrogen to find its partial pressure:
Mole Fraction nitrogen:
7.00 moles Nitrogen / (7.00moles N2 + 2.50moles O2 + 0.500moles He) = 0.700 = Mole fraction.
Partial Pressure = 0.700* 0.900atm
Partial Pressure = 0.630atm