Answer the following questions. Using the information on the
picture below. Thank you
1. What are the reactants in this experiment?
2. What are the products in this experiment?
3. Using the proced
Vinegar and Baking Soda Stoichiometry Lab Introduction In this lab, we will be reacting vinegar and baking soda to determine the mass of carbon dioxide produced during the reaction. We will use this m
Answers
1. The reactants in this experiment are vinegar and baking soda. 2. The products in this experiment are water, carbon dioxide, and sodium acetate.
1. The reactants in this experiment are vinegar and baking soda. Vinegar is a solution of acetic acid in water. It is an acidic substance with a sour taste and pungent smell. Baking soda is a white crystalline solid that is also known as sodium bicarbonate. It is a basic substance that reacts with acids to produce carbon dioxide gas.
2. The products in this experiment are water, carbon dioxide, and sodium acetate. When vinegar and baking soda are mixed, a chemical reaction occurs. The acetic acid in the vinegar reacts with the sodium bicarbonate in the baking soda to produce carbon dioxide gas, water, and sodium acetate.
The balanced chemical equation for this reaction is as follows: CH3COOH + NaHCO3 → NaC2H3O2 + CO2 + H2O. The carbon dioxide gas produced during the reaction is what we will be measuring in this lab. We will do this by collecting the gas in a balloon and measuring the mass of the balloon before and after the reaction. By subtracting the mass of the balloon from the mass of the balloon and gas, we will be able to determine the mass of carbon dioxide produced during the reaction.
To know more about carbon dioxide visit :
https://brainly.com/question/3049557
#SPJ11
Related Questions
what are 7 reasons viruses can be classified as nonliving and 3 for them for being classified as living?
Answers
Answer:
Im not sure if they're all right but i hope this helps <3
Explanation:
nonliving:
- it doesn't need to consume energy to survive
- its not able to regulate its temperature
- they replicate by invading living cells
- living things have cells whereas viruses do not
- they aren't made of living cells
- they don't produce waste products
- they don't grow
living:
- they can adapt to their environment
- living things have different levels of organization and so do viruses
- viruses can infect other viruses. If a virus can get sick it should be considered a living thing.
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
How do renewable and nonrenewable resources differ?
Answers
Natural resources are those that are accessible without human intervention. Like the sun, the atmosphere, the air, the water, the land, the mines, the vegetation, and animal life.
There are two categories of natural resources: renewable resources and non-renewable resources.Renewable resources :
These are the kind of natural resources that, even after constant use, do not become exhausted or depleted.For example, Wind and SunlightThese have low carbon footprints and low carbon emissions.Infrastructure costs for the production of renewable energy are very costly.Doesn't cause Pollution. Cause Pollution when usedNon Renewable resources :
These are the natural resources that, as a result of ongoing human usage, become exhausted or depleted and are neither renewed nor replaced.For example, groundwater, fossil fuels, and mineral ores etc.These emit more carbon than other, which increases their carbon footprint.Infrastructure costs for the production of energy from these resources are low.Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ9
Which material is the least likely to be recognized as a mixture by looking at it under a microscope? a suspension a colloid a homogeneous mixture a heterogeneous mixture
Answers
Answer:
A homogeneous mixture for sure
Homogenous mixture material is the least likely to be recognized as a mixture by looking at it under a microscope.
What is homogenous mixture?A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture and we can not see the solute and solvent separately so we can conclude that Homogenous mixture material is the least likely to be recognized as a mixture by looking at it under a microscope.
Learn more about mixture here: https://brainly.com/question/24647756
Question 10 (1 point)
Which of the following is NOT a way substance properties are used?
O a
b
O C
Od
A-To figure out the density or boiling/melting points of the materials.
B-To help us determine what materials can be used for.
C-To separate mixtures.
D-To identify the materials.
Answers
Answer:
B
Explanation:
Due to the fact that by us knowing that H2O is water, it doesn't tell us that we can drink it.
does that make sense to you, if not, I can give you another example.
12579 nm rounded to 3 significant figures is
Answers
\(\\ \sf\longmapsto 12579nm\)
\(\\ \sf\longmapsto 12579\times 10^{-9}m\)
\(\\ \sf\longmapsto 1257.9\times 10^{-8}m\)
\(\\ \sf\longmapsto 12.5\times 10^{-6}m\)
\(\\ \sf\longmapsto 12.5\mu m\)
Answer:
1.26e-5
Explanation:
Change 12579 nm to meters first, it becomes 1.2579e-5, then rounding it to 3 significant figures becomes 1.26e-5.
The specific heat capacity of liquid water is 4.18 J/g-K. How many joules of heat are needed to raise the temperature of 5.00 g of water from 15.0 °C to 36.5 °C?
Answers
The specific heat capacity of a substance is the amount of energy required to raise the temperature of 1g of the substance by 1K.
\(\begin{gathered} q=mc\Delta T \\ q:energy\text{ }(J)=x \\ m:mass\text{ }(g)=5.00g \\ c:specific\text{ }heat\text{ }capacity\text{ }(Jg^{-1}K^{-1}) \\ \Delta T:change\text{ }in\text{ }temperature\text{ }(K) \\ \Delta T:(final\text{ }temperature-initial\text{ }temperature) \end{gathered}\)Calculating the change in temperature:
\(\Delta T:(273.15K+36.5\degree C)-(273.15K+15\degree C)=21.5K\)By substituting what we are given into the equation to solve for the unknow x we have;
\(\begin{gathered} q=5.00g\times4.18Jg^{-1}K^{-1}\times21.5K \\ q=+449.35J \end{gathered}\)Answer: Energy needed is 449.35J
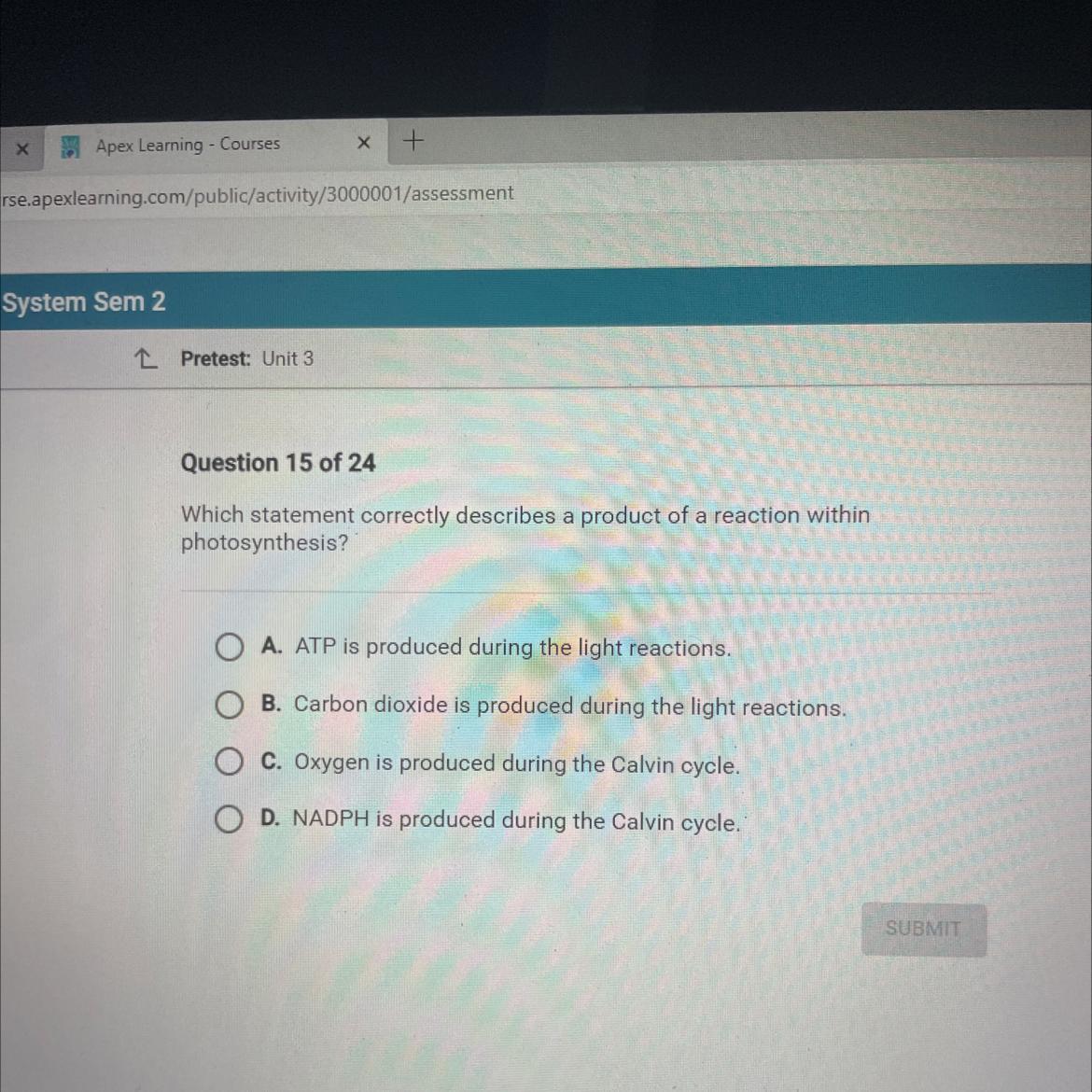
Which statement correctly describes a product of a reaction within
photosynthesis?
O A. ATP is produced during the light reactions.
O B. Carbon dioxide is produced during the light reactions.
O C. Oxygen is produced during the Calvin cycle.
OD. NADPH is produced during the Calvin cycle.
Answers
Answer:
B
pls correct me if im wrong
Three balloons filled with neon, carbon dioxide, and hydrogen (respectively) are released into the
atmosphere. Using the data in Table 3.6 on page 90, describe the movement of each balloon.
Answers
Three balloons filled with neon carbon dioxide and hydrogen are released into the atmosphere using the data in Table 3.6 on page 90 describe the movement of each balloon
Three balloons filled with neon, carbon dioxide, and hydrogen are released into the atmosphere. ... The neon and hydrogen balloons will rise/ float because they have a lighter density than air. The carbon dioxide balloon will sink/not rise because it has a heavier density than air.
we dissolve 2.45 g of sugar in 200.0 g water. what is the mass percent of sugar in the solution? we dissolve 2.45 g of sugar in 200.0 g water. what is the mass percent of sugar in the solution? 1.21% 123% 1.23% 2.42% none of the above
Answers
When 2.45 grams of sugar are dissolved in 200.0 grams of water, the mass percentage of sugar in the solution is 1.21%.
Mass Percent-
The concentration of chemical substances in solutions can be stated in a variety of ways. While the molar concentration refers to the quantity of moles of the solute in the solution, the mass concentration refers to the mass of the solute per unit volume of the solution. The mass percent of the solute in the solution is expressed as a percentage. While molar and mass concentrations have units of measurement, mass percent has none.
The total mass is 202.45 gms when 2.45 grams of sugar are dissolved in 200.0 grams of water. According to the definition and idea of the mass percent, such a solution would have a sugar content of (2.45 /202.45) 100 = 1.21% sugar.
To know more about units,
https://brainly.com/question/28464
#SPJ4
calculate the density of a liquid with a total mass of 88.30 grams and a volume of 165.0 milliliters.
Answers
The liquid with a total mass of 88.30 grams and a volume of 165.0 milliliters has a density of: 0.5351 g/ml
To solve this problem the formula and the procedure that we have to use is:
d = m/v
Where:
d= densitym= massv= volumeInformation about the problem:
m = 88.30 gv= 165.0 mld=?Applying the density formula we get:
d = m/v
d = 88.30 g/165.0 ml
d = 0.5351 g/ml
What is density?It is a physical quantity that expresses the ratio of the body mass to the volume it occupies.
Learn more about density in: brainly.com/question/1354972
#SPJ4

Brownian motion is
A. random movement of particles suspended in a fluid
B. movement of particles from an area of high concentration to low concentration
C. movement of particles from an area of low concentration to high concentration
D. random movement of smaller particles
Answers
Answer: A.
Explanation:
Brownian motion is the random motion of a particle as a result of collisions with surrounding gaseous molecules. Diffusiophoresis is the movement of a group of particles induced by a concentration gradient. This movement always flows from areas of high concentration to areas of low concentration.
Example: The movement of pollen grains on still water. Motion of dust motes in a room (though largely influenced by air currents).
How many moles of Au are in 312 g of Au?
Answers
Answer:
1.583 moles
Explanation:
Rounded Atomic Mass of Au = 197 grams
\(\frac{312}{197} =1.5837, 1.584\)
Is HCL considered polar or nonpolar?
Answers
HCL is classified as a polar molecule. The distribution of electrical charge within a molecule is referred to as polarity. The electrons in a polar molecule are not equally distributed, and there is charge separation. This can be caused by a variation in electronegativity, which is a measure of how strongly an atom attracts electrons, between the atoms in a molecule.
The electronegativity of chlorine is significantly higher than that of hydrogen, implying that the chlorine atom attracts electrons far better than the hydrogen atom. As a result of the charge separation within the molecule, HCL becomes a polar molecule.
This polarity is also demonstrated by the angle of the H-Cl bond, which is approximately 107.5 degrees. Because this angle is not 180 degrees, electrons are distributed unevenly, making it polar.
For more such questions on Polarity.
https://brainly.com/question/3184550#
#SPJ11
Research the compositions of Pennies. What was the composition of each of your Pennies prior to treatment
Answers
Answer:
History of composition
Years Material Weight (grains)
1944–1946 gilding metal (95% copper, 5% zinc) 48 grains
1947–1962 bronze (95% copper, 5% tin and zinc) 48 grains
1962 – September 1982 gilding metal (95% copper, 5% zinc) 48 grains
October 1982 – present copper-plated zinc (97.5% zinc, 2.5% copper) 38.6 grains
Which formula represents a
polar molecule
containing polar covalent bonds?
A. H2O
B. CO2
C. NaCL
D. Cl2
Answers
Answer:
A. H2O
Explanation:
Let us first define the three types of bonds:
1. Nonpolar Covalent: electronegativity difference < 0.4
2. Polar Covalent: electronegativity difference between 0.4 and 1.8
3. Ionic: electronegativity difference > 1.8
This will help us eliminate choices C and D:
-NaCl has a electronegativity difference of 3.0 - 0.9 = 2.1 (ionic bond)
-Cl2 has a electronegativity difference of 3.0 - 3.0 = 0 (nonpolar covalent bond)
However, we still have two more options, A and B, but they are not diatomic for us to use the electronegativity differences with.
We must now consult their geometries. Because CO2 has a linear geometry (O=C=O), the two sides will cancel each other out, resulting in a nonpolar covalent bond. At this point, by process of elimination, we can already determine the answer to be A. H2O. We can verify this by looking at the geometry of H2O, which is bent (H-O-H; imagine the O is above the H's, I cannot draw it in this response). H2O's bent geometry classifies it as polar covalent; the electrons are slightly more attracted towards the O, the more electronegative element. Side note: this makes the O slightly more negative in charge, whilst the H's are slightly more positive in charge.
P.S. I apologize for not being able to draw and demonstrate that last paragraph, but I hope you get a general idea. You can search up the "H2O geometry" and "CO2 geometry" to get a better idea! :)
H₂O represents a nonpolar molecule containing polar covalent bonds. Hence Option (A) is correct
What is Polar Covalent Bond ?A polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond.
Consider the hydrogen chloride (HCl) molecule. Each atom in HCl requires one more electron to form an inert gas electron configuration.
Water (H₂O), like hydrogen Chloride (HCl), is a polar covalent molecule. When we look at a diagram of water , we can see that the two hydrogen atoms are not evenly distributed around the oxygen atom
Water (H₂O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.Thus, This is an example of polar covalent chemical bonding.
Therefore, H₂O represents a nonpolar molecule containing polar covalent bonds. Hence Option (A) is correct
Learn more about bonding here :
brainly.com/question/1603987
#SPJ2
im giving out the game the last of us part 2 who wants it
Answers
Answer:
YEESSS
Explanation:
Because that seems like the only logical answer.
Answer:
YES
Explanation:
YES
Why would writing the chemical formula only in its simplest form (empirical formula)
cause confusion when discussing a chemical molecule?
Answers
Writing the chemical formula only in its simplest form (empirical formula) helps to represent the relative proportions of atoms in a compound. It is useful for identifying the identity of an unknown compound and for predicting its properties.
How does the empirical formula differ from the molecular formula?The empirical formula represents the simplest whole-number ratio of atoms in a compound, whereas the molecular formula specifies the actual number of each type of atom in a molecule.
The empirical formula is useful for identifying the identity of an unknown compound and for predicting its properties.
Can the empirical formula be used to determine the molecular formula of a compound?Yes, the empirical formula can be used to determine the molecular formula of a compound, but additional information is needed, such as the molar mass of the compound.
Once the molar mass is known, the empirical formula can be used to calculate the molecular formula by determining the number of empirical formula units that would be needed to reach the molar mass.
To know more about chemical formula,visit:
https://brainly.com/question/29031056
#SPJ1
To describe the relative proportions of atoms in a compound, the chemical formula should only be written in its simplest form (empirical formula). It is helpful for figuring out a compound's identification and foretelling its properties.
What distinguishes the molecular formula from the empirical formula?The empirical formula defines the precise number of each type of atom in a molecule, whereas the empirical formula represents the simplest whole-number ratio of atoms in a compound.
The empirical formula can be used to determine a compound's identity and forecast its properties.
Can the molecular formula of a substance be determined using the empirical formula?The empirical formula can be used to ascertain the molecular formula of a combination, but other details, such as the compound's molar mass, are required.
The empirical formula can be used to determine the molecular formula once the molar mass is known by counting the number of empirical formula units required to get there.
To know more about chemical formula,visit:
brainly.com/question/29031056
#SPJ1
if lead metal is added to a 0.100 m cr3 (aq) solution. what are the concentrations of pb2 (aq), cr2 (aq), and cr3 (aq) when the reaction is at equilibrium?
Answers
The concentrations of Pb₂⁺ and Cr₂⁺ at equilibrium are 0 M and 0.100 M, respectively. The concentration of Cr₃⁺ at equilibrium is 0.200 M.
To determine the concentrations of the different species at equilibrium, we need to write the balanced chemical equation for the reaction between lead metal and chromium(III) ion:
Pb(s) + 2Cr₃⁺(aq) → Pb₂⁺(aq) + 2Cr₂⁺(aq)
We also need to know the initial concentration of chromium(III) ion, which is given as 0.100 M.
Since lead metal is a solid, it does not have a concentration. Instead, its presence affects the equilibrium concentrations of the other species. We assume that the reaction goes to completion, which means that all the chromium(III) ion will react with the lead metal.
At equilibrium, we can use an ICE table to determine the concentrations of the different species:
Initial: 0.100 M 0 M 0 M
Change: -0.100 M +0.100 M +0.200 M
Equilibrium: 0 M 0.100 M 0.200 M
At equilibrium, the concentrations of Pb₂⁺ and Cr₂⁺ are 0 M and 0.100 M, respectively. At equilibrium, the concentration of Cr₃⁺ is 0.200 M.
To know more about the Equilibrium, here
https://brainly.com/question/12676702
#SPJ4
A) Calculate the percent ionization of formic acid (HCO2H) in a solution that is 0.311 M in formic acid and 0.189 M in sodium formate (NaHCO2). The Ka of formic acid is 1.77 × 10-4. ( Answer:0.0952)
B)5) Suppose you have just added 100.0 ml of a solution containing 0.5000 moles of acetic acid per liter to 400.0 ml of 0.5000 M NaOH. What is the final pH? The Ka of acetic acid is 1.770× 10-5. ( Answer:13.48)
Answers
A) The percent ionization of formic acid is 0.0952.
B) The final pH is 13.48.
How to calculate percent ionization and final pH?Explanation for Part A:
When calculating the percent ionization of formic acid (HCO₂H), we consider the concentration of H⁺ ions compared to the initial concentration of formic acid. In this scenario, we have a solution with a concentration of 0.311 M formic acid and 0.189 M sodium formate (NaHCO₂). Since sodium formate dissociates completely, it serves as a source of H+ ions.
In this case, we can assume that the contribution of H⁺ ions from sodium formate is significant compared to the ionization of formic acid. Therefore, the concentration of H⁺ ions is equal to the concentration of sodium formate, which is 0.189 M.
To calculate the percent ionization, we use the formula:
percent ionization = (concentration of H⁺ ions / initial concentration of formic acid) x 100
Substituting the values, we have:
percent ionization = (0.189 / 0.311) x 100 = 0.0952 x 100 = 9.52%
Therefore, the percent ionization of formic acid in the given solution is 0.0952 or 9.52%.
Explanation for Part B:
When mixing acetic acid (CH₃COOH) with sodium hydroxide (NaOH), a neutralization reaction occurs. Acetic acid is a weak acid, while sodium hydroxide is a strong base. The reaction between the two results in the formation of water and sodium acetate (CH₃COONa).
To determine the final pH, we need to consider the reaction and the resulting species in the solution. In this case, we have added 100.0 ml of a solution containing 0.5000 moles of acetic acid per liter to 400.0 ml of 0.5000 M NaOH.
Since acetic acid is a weak acid, we can assume that its ionization is negligible compared to the complete dissociation of NaOH. Therefore, we can consider the solution as a strong base solution.
When a strong base reacts with water, it produces hydroxide ions (OH⁻) which leads to an increase in the concentration of OH⁻ ions. To determine the pH, we need to calculate the concentration of OH⁻ ions, which can be done by considering the moles of NaOH and the total volume of the solution.
Using the given values, we have:
Moles of NaOH = 0.5000 M x 0.4000 L = 0.2000 moles
Total volume of the solution = 0.1000 L + 0.4000 L = 0.5000 L
Concentration of OH⁻ ions = moles of NaOH / total volume of the solution
= 0.2000 moles / 0.5000 L
= 0.4000 M
Since pH is defined as the negative logarithm (base 10) of the concentration of H⁺ ions, we can use the pOH to determine the pH. The pOH is equal to -log10[OH⁻] in this case.
pOH = -log10[0.4000] = 0.3979
Finally, we can determine the pH using the relationship between pH and pOH:
pH = 14 - pOH = 14 - 0.3979 = 13.6021 ≈ 13.60
Learn more about ionization
brainly.com/question/1602374
#SPJ11
6. ( i’ll award the brainliest and any “links” will be reported)
sodium metal reacts with water and produces aqueous sodium hydroxide and hydrogen gas
a. Translate the word equation, and write the complete and balanced chemical equation.
b. What volume of hydrogen gas can be produced from reacting 5.67 grams of sodium metal with excess
water?
C. What is the percent yield if 964.00 mL of H, are collected through experimentation?
Answers
Answer: 2Na+2H2O------>2NaOH+H2
b) From the equation two moles of sodium gave one mole of hydrogen.
At stp the volume of hydrogen is 22400cm3.
The molar mass of sodium is 23
So we have
2molesNa--->1moleH2
5.76moles--->? mole H2
Meaning
2(23)Na----> 22400cm3 ofH2
5.67Na---->?H2
22400×5.67÷ 2(23)
127008÷46=2761.04cm3
Sorry I can't do the last one.
A hydrocarbon fuel is fully combusted with 18.214 g of oxygen to yield 23.118 g of carbon dioxide and 4.729 g of water. Find the empirical formula for the hydrocarbon. * o CH o CH2
o C2H2 o C2H3
Answers
The empirical formula is therefore CH.Now we can calculate the empirical formula. Divide the number of moles of each element by the smallest number of moles to get a simple whole number ratio:
Carbon: 0.5250 mol C / 0.5250 mol = 1
Hydrogen: 0.5242 mol H / 0.5250 mol = 0.997
To find the empirical formula for the hydrocarbon, we need to determine the moles of each element present in the combustion reaction.
First, let's determine the moles of oxygen used:
18.214 g of O2 x 1 mol O2/32.00 g = 0.5698 mol O2
Next, let's determine the moles of carbon dioxide produced:
23.118 g of CO2 x 1 mol CO2/44.01 g = 0.5250 mol CO2
Finally, let's determine the moles of water produced:
4.729 g of H2O x 1 mol H2O/18.02 g = 0.2621 mol H2O
To find the moles of carbon present in the hydrocarbon, we can use the fact that the amount of carbon in the hydrocarbon equals the amount of carbon in the carbon dioxide:
0.5250 mol CO2 x (1 mol C/1 mol CO2) = 0.5250 mol C
To find the moles of hydrogen present in the hydrocarbon, we can use the fact that the amount of hydrogen in the hydrocarbon equals the amount of hydrogen in the water:
0.2621 mol H2O x (2 mol H/1 mol H2O) = 0.5242 mol H
Now we can calculate the empirical formula. Divide the number of moles of each element by the smallest number of moles to get a simple whole number ratio:
Carbon: 0.5250 mol C / 0.5250 mol = 1
Hydrogen: 0.5242 mol H / 0.5250 mol = 0.997
The empirical formula is therefore CH.
learn more about formula for the hydrocarbon here
https://brainly.com/question/30884168
#SPJ11
How do you cook a spiral ham without drying it out?.
Answers
The best way to cook a spiral ham without drying it out is to use the low and slow method.
What is method ?A method is a procedure or a technique used to produce the intended results. It is a methodical technique to problem solving that entails dividing a task into smaller components and carrying them out in a specified manner.
Methods are employed in every aspect of life, including commerce, engineering, and mathematics. In the sciences, where the scientific method is applied to test hypotheses and derive conclusions, methods are particularly crucial.
This entails cooking the gammon for a longer amount of time (approximately 15 minutes per pound) at a low temperature (about 325°F). Remove the gammon from its plastic wrapper before cooking it, and set it in a shallow roasting pan.
After that, cover the ham with foil, making sure that it is tightly sealed. Then, place the ham in the oven and cook it for the recommended length of time. Lastly, about 10 minutes before the end of the cooking time, remove the foil and brush the ham with a glaze of your choosing. This will help add flavor and moisture to the ham and help keep it from drying out.
To learn more about spiral ham
https://brainly.com/question/30518035
#SPJ4
The size of an atom generally increases down a group and from right to left across a period. up a group and from left to right across a period. down a group and from left to right across a period up a group and from right to left across a period. up a group and diagonally across the Periodic Table. Which set shows the correct resonance structures for SeO_2? SeO_2 does not have a resonance structure. Which of the following ions doesn't have the same electronic configuration noble gas? Cl_- N^3+ S^2- So^3+ None of the above The bond length of 1.27 Angstrom, what is the dipole moment in debayes, if the charges on H and Cl were +1 and -, respectively? 4.79 D 1.63 D 6.08 D 1.08 D None of the above What is the estimation of the delta H (Bond dissociation energy change) for the following gas phase reaction? CHBr_2 + Cl_2 rightarrow CBr_3Cl + HCl D(C-H) = 413kj, D(Cl-Cl) = 242 kJ, D(C-Cl) = 328 kJ, D(H-Cl) = 43kJ.
Answers
Size of an atom increases as we move down a group and from left to right across a period
Define an atom?
An atom is a unit of matter that specifically characterizes a chemical element. One or more negatively charged electrons surround the core nucleus of an atom, which is made up of all of them. One or more protons and neutrons, which are comparatively heavy particles, can be found in the positively charged nucleus.
In a group, as the atomic number rises, the atomic size expands from top to bottom. Valence electrons are located farther from the nucleus because there are more filled energy levels, which increases atomic size.
Atomic size grows as a function of period number, number of shells, and so forth. Since there are more electrons in each shell as we move from left to right in a period, the force of attraction between the nucleus and electrons, which have positive charges, is stronger, bringing the shells closer to the nucleus and shrinking the size of the atom.
To know more about atomic number use link below:
https://brainly.com/question/11353462
#SPJ1
Sig fig 35 mm + 21.321 mm + 2.00005 mm =
Answers
Answer:
Significant Figures in 200.0
Result 200.0
Sig Figs 4 (200.0)
Decimals 1 (200.0)
Scientific Notation 2.000 × 102
E-Notation 2.000e+2
if a sample of rock contains 0.009 percent gold by mass, how much nacn is needed to extract the gold
Answers
A particular ore employed in this study needed a concentration of 100 g/L (200 lb/st solution or 600 lb/st ore) NaCN to extract gold at a rate of over 90 percent.
In how much cyanide does gold mining take place?Since the 1970s, cyanide leaching, often known as "cyanidation," has been the main method used to extract gold. In this method, sodium cyanide is used to selectively dissolve gold from ore in a solution that contains between 100 and 500 parts per million, or 0.01% and 0.05% cyanide.
Where is cyanide most prevalent?Certain bacteria, fungus, and algae can generate cyanides. Additionally, cyanides can be found in things like tapioca, spinach, bamboo shoots, almonds, lima beans, fruit pits, and car exhaust.
To know more about extract of gold visit:-
https://brainly.com/question/7719613
#SPJ4
Which elements have similar behavior? barium silicon aluminum strontium osmium beryllium
Answers
Answer:
Barium, beryllium, and Strontium
Explanation:
These 3 elements are located in the same group, which determines the number of valence electrons.
The valence electrons determine how reactive an element will be.
A. →barium
B. silicon
C. aluminum
D. →strontium
E.osmium
F. →beryllium
Hope that this helps! :) :) :) :) :)
A 32.44 g sample of iron chloride is found to contain 14.29 g of iron. What is the mass percent of the iron in the sample? Calculations?
Answers
The mass percent of iron in 32.44 grams of iron chloride is 44.05%.
How to calculate the mass percent?The mass percent of an element in a compound can be calculated by dividing the mass of element by the mass of the compound, then multiplied by 100 as follows:
mass percent = mass of iron/mass of iron chloride × 100
According to this question, 32.44 g sample of iron chloride is found to contain 14.29 g of iron. The mass percent can be calculated as follows:
% percent = 14.29g/32.44g × 100
% percent = 44.05%
Therefore, 44.05% is the mass percent of the iron in the sample.
Learn more about mass percent at: https://brainly.com/question/5394922
#SPJ1
if 4.95 g of ethylene (c2h4) are combusted with 3.25 g of oxygen. first write the balanced equation a. what is the limiting reagent? a. how many grams of co2 are formed?
Answers
The limiting reagent is oxygen .
The mass of carbon dioxide produced is 2.9612g.
What is limiting agent ?
When a chemical reaction is complete, the limiting reagent—also known as the limiting reactant or limiting agent—is the reactant that has been completely consumed.
Ethane, often known as ethylene, is an unsaturated carbon molecule made up of two carbon atoms. Ethylene is burned in the presence of oxygen during the combustion reaction, producing carbon dioxide and water as byproducts.
Complete response:
Hydrocarbons are burned when they come into contact with oxygen, which causes a chemical reaction that produces carbon dioxide and water. The following is a representation of the balanced chemical equation for ethylene combustion:
C2H4+3O2→2CO2+2H2O
According to stoichiometric coefficients, one mole of ethylene produces two moles of carbon dioxide gas and two moles of water vapour for every mole of oxygen that is used.
Ethylene and oxygen therefore react at a 1:3 ratio.
The ratio of their masses to their molar masses can be used to calculate the amount of moles of ethylene and oxygen.
given mass = n
in molar mass
The number of ethylene moles is:
n(C2H4)=4.95g28gmol−1=0.177
The formula for oxygen moles is n(O2)=3.25g32gmol1=0.101
A reactant that is less prevalent and is consumed earlier in the reaction is the limiting reagent.
Although there is less oxygen available for the reaction, 0.1773=0.531g of oxygen is required to maintain a 1:3 ratio between ethylene and oxygen. So, the limiting reagent is oxygen.
The amount of limiting reagents will determine how the reaction proceeds since once they are used up, the reaction ceases.
As a result, the ratio of oxygen reacting and carbon dioxide being created is 3:2, resulting in the formation of two-thirds (23) of a mole of carbon dioxide for every mole of oxygen.
As a result, two-thirds more moles of carbon dioxide were created than were used in the reaction of oxygen:
n(CO2)=0.101×(23)=0.0673
By dividing the moles by the molar mass of carbon dioxide, it is possible to determine the mass of carbon dioxide that is created.
n(CO2)=0.0673mol×44gmol−1=2.9612g
Therefore,
(a) oxygen is the limiting reagent and
(b) 2.9612g of carbon dioxide are generated.
Learn more about limiting agent from given link
https://brainly.com/question/26905271
#SPJ4
Help me with this please

Answers
Answer:
–253.5 °C
Explanation:
We'll begin by calculating the number of mole in 6 g of CO₂. This can be obtained as follow:
Molar mass of CO₂ = 12 + (2×16)
= 12 + 32
= 44 g/mol
Mass of CO₂ = 6 g
Mole of CO₂ =.?
Mole = mass / molar mass
Mole of CO₂ = 6 / 44
Mole of CO₂ = 0.136 mole
Next, we shall convert 225 mL to L.
1000 mL = 1 L
Therefore,
225 mL = 225 mL × 1 L / 1000
225 mL = 0.225 L
Next, we shall determine the temperature of the gas. This can be obtained as follow:
Pressure (P) = 0.855 atm
Volume (V) = 0.225 L
Number of mole (n) = 0.136 mole
Gas constant (R) = 0.0821 atm.L/Kmol
Temperature (T) =?
PV =nRT
0.855 × 0.255 = 0.136 × 0.0821 × T
0.218025 = 0.0111656 × T
Divide both side by 0.0111656
T = 0.218025 / 0.0111656
T = 19.5 K
Finally, we shall convert 19.5 K to degree celsius (°C). This can be obtained as follow:
T(°C) = T(K) – 273
T(K) = 19.5 K
T(°C) = 19.5 – 273
T(°C) = –253.5 °C
Therefore, the temperature of the gas is –253.5 °C