Arrange this isoelectronic series in order of decreasing radius: F-02-, Mg2+ , Na+ .
Answers
Answer:
Mg2+ < Na+ <F−< O2−
Explanation:
For isoelectronic species, ionic radii decrease with an increase in nuclear charge (i.e., no. of protons). Thus, for isoelectronic species, the cation with the greater +ve charge will have a smaller radius and the anion with greater -ve charge will have a larger radius.
thus, the answer is Mg2+ < Na+ <F−< O2−
Related Questions
how many grams of sucrose, c12h22o11, must be added to 500 g of water at 100c to change the vapor pressure to 752 mmhg?
Answers
To change the vapor pressure to 752 mmHg, 27.8 g of sucrose (C12H22O11) must be added to 500 g of water at 100C.
Vapor pressure of a solution is affected by the presence of solutes in the solution. The relationship between vapor pressure lowering and concentration of a non-volatile solute in a solvent is described by Raoult's law.
It states that the vapor pressure of a solution is equal to the mole fraction of the solvent multiplied by the vapor pressure of the pure solvent, P₀. Hence, Pᵥ = P₀ x Xw
where, Pᵥ = vapor pressure of the solution Xw = mole fraction of the solvent P₀ = vapor pressure of the pure solvent
Therefore, P₀ - Pᵥ = P₀ x (1 - Xw)
This equation can be used to calculate the vapor pressure lowering of a solution relative to the pure solvent. By definition, the mole fraction of the solvent is given by
Xw = number of moles of solvent / total number of moles of solute and solvent.
Since we assume that the volume of the solution is 500 g of water + m g of sucrose, where m is the mass of sucrose, we can write
0.752 atm x 760 mmHg / atm = P₀ x (500 g / (500 g + m))
m= 27.8g
Therefore, we have m = 27.8 g of sucrose.
To know more about vapor pressure, refer here:
https://brainly.com/question/11864750#
#SPJ11
Where are most volcanoes located? (Use information from the map.)
What is happening to the earth’s crust in these locations?
Answers
Every atom of the __ carbon has six protons
Answers
9. How many grams of zinc are needed to produce 12 grams of zinc chloride according to the following equation? Y-
Zn + Cl2 --> ZnCl2
12 grams of zinc
O 2.9 grams of zinc
O 11.6 grams of zinc
O 5.8 grams of zinc
Answers
Given :
A balanced chemical equation :
\(Zn +Cl_2->ZnCl_2\)
To Find :
How many grams of zinc are needed to produce 12 grams of zinc chloride.
Solution :
Moles of \(ZnCl_2\) ,
\(n=\dfrac{Given \ wt}{Molecular\ Mass}\\\\n =\dfrac{12}{136.30}\ mol\\\\n=0.088\ mol\)
Now, by balanced chemical equation we can say that 1 mol of Zn produce
1 mol of \(ZnCl_2\) .
So, 0.088 mol of Zn is required to produced 0.088 mol of \(ZnCl_2\) .
\(Mass \ required = molecular \ mass \times moles\\\\m = 65.38 \times 0.088\\\\m=5.8 \ gm\)
Therefore, 5.8 grams of zinc is required.
Hence, this is the required solution.
Calcium is a group 2 element. Chlorine is a group 7 element. They form an ionic compound called
calcium chloride. In calcium chloride, for every one atom of calcium, how many atoms of chlorine will
there be?
HELPPPPP
Answers
Answer:
CaCl2
Explanation:
For every calcium there's 2 chlorine
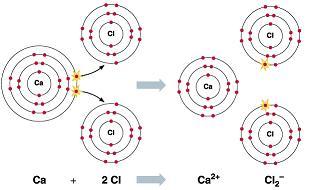
There are two types of chemical compound one is covalent compound and another is ionic compound in chemistry. In ionic bonds, electrons are completely transferred. Therefore, for every calcium there are 2 chlorine atoms.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
Covalent compounds are formed by covalent bond and ionic compounds are formed by ionic bond. Covalent bond is formed by sharing of electron and ionic bond are formed by complete transfer of electron. Ionic bonds are stronger than covalent bonds. The melting and boiling points are higher in ionic compounds.
In CaCl\(_2\), the subscript 2 represents the number of atoms of chlorine. For every calcium there are 2 chlorine atoms.
Therefore, for every calcium there are 2 chlorine atoms.
To learn more about chemical compound, here:
https://brainly.com/question/26487468
#SPJ2
Brian's family has a compost pile at home. They put all the weeds they pull up from the garden in it as well as any vegetable waste they gather from the kitchen. If they keep it moist, all this vegetation will
eventually turn into organic soil by the chemical process of decay. Since decay involves a variety of chemical reactions, how might temperature affect the rate at which the compost turns into sol?
Temperatures near freezing would make it speed up.
It would happen faster at warmer air temperatures.
Changing temperatures would not affect the chemical reactions.
Warm summer air temperatures would make it slow down.
Answers
Answer:
Option - It would happen faster at warmer air temperatures
Explanation:
The chemical process of converting organic matter such as plants, litter into organic soil, or organic compost called decomposition and perform by decomposers such as fungus or bacteria.
Like all other living organisms, decomposers also live in an optimal range of temperature. The major decomposers normally lie in the range of 30 to 40-degree celsius. Any type of fluctuation in this range might slow down the reaction of decomposition.
Convert the following to Celsius
6) 32°F
7) 45°F
8) 70°F
9) 80° F
10) 90° F
11) 212°F
Answers
9. (80-32)5/9
48×5/9
240/9
26.66°C
9. (90-32)5/9
58×5/9
32.22°C
10 (212-32)5/9
180×5/9
20×5
100°C

Write and balance the following single
replacement reaction.
g) Ca + H₂O (steam)
Answers
The balanced equation for this single replacement reaction is Ca + 2H₂O → Ca(OH)₂ + H₂.
The products like Calcium (Ca) and water (H₂O) are reacting with each other and they are balanced by using the appropriate coefficient. When calcium interacts with water, it goes through an oxidation process that removes the hydrogen from the reduced water molecule.
The reaction is a single replacement reaction because as we can see, the ions of one of the species in the products are changed only. About the balancing part, we have to make sure that the reaction follow the law of conservation of mass.
To know more about single replacement reaction, visit,
https://brainly.com/question/28909116
#SPJ1
Does the physical form of the material matter for mass-mole
and mole-mass calculations?
Answers
Sn(s)+2HF(g)→SnF2(s)+H2(g)
Sn(s)+2HF(g)→
SnF
2
(s)+
H
2
(g)
How many moles of hydrogen fluoride are required to react completely with 75.0 g of tin?
Step 1: List the known quantities and plan the problem.
Known
given: 75.0 g Sn
molar mass of Sn = 118.69 g/mol
1 mol Sn = 2 mol HF (mole ratio)
Unknown
mol HF
Use the molar mass of Sn to convert the grams of Sn to moles. Then use the mole ratio to convert from mol Sn to mol HF. This will be done in a single two-step calculation.
g Sn → mol Sn → mol HF
Step 2: Solve.
75.0 g Sn×1 mol Sn118.69 g Sn×2 mol HF1 mol Sn=1.26 mol HF
75.0 g Sn×
1
mol Sn
118.69
g Sn
×
2
mol HF
1
mol Sn
=1.26 mol HF
Step 3: Think about your result.
The mass of tin is less than one mole, but the 1:2 ratio means that more than one mole of HF is required for the reaction. The answer has three significant figures because the given mass has three significant figures.
The physical form of the material matter for mass-mole and mole-mass calculations is solid.
What is the relation between mass & moles?Relation between mass and moles will be represented as:
n = W/M, where
W = given mass in grams
M = molar mass in grams/mole
So the physical state of the matter that is used in the above relation is solid because solid is only present in the grams and kilogrmas quantity.
Hence solid is the physical form of the matter.
To know more about mass & moles, visit the below link:
https://brainly.com/question/15373263
#SPJ2
The Kinetic Molecular Theory describes the behavior as well as the characteristics of an ideal gas. What are the five postulates of this theory? Provide at least 3 examples to describe these postulates.
Answers
Answer:
See explanation
Explanation:
The postulates of the kinetic theory of matter are;
Every substance is made up of tiny particles called molecules. Brownian motion and diffusion illustrates this fact.The molecules that compose matter are in constant random motion.There exists an attractive force between the molecules in matter. The attractive forces between gases are negligible. Solids have a definite shape and volume due to a high magnitude of intermolecular forces. Liquids have a volume but no definite shape due to weaker intermolecular forces. Gases have the weakest intermolecular forces hence the do not have both a shape and volume. They take on the volume of the container into which they are put. This illustrates this fact.The actual volume occupied by gas molecules is negligible relative to the volume of the container. The fact that gases are easily compressible illustrates this fact.Temperature is a measure of the average kinetic energy of the molecules of a body.would you expect the substance to float or sink in water if mass is 25g and volume is 7 cm
Answers
Answer:
Sink
Explanation:
For a substance to float in water, it must be less dense than water. The density of water is 1 g/cm^3. To find the density of the substance, we can do mass/volume, which in this case is 25g/7cm^3. We see that \(25/7 > 1\), so the substance would sink.
WILL GIVE BRAINLIEST!!!!!!!!!!!!
Consider the diagram below.
What best describes this diagram?
A) building blocks for creating fats
B) building blocks for creating proteins
C) building blocks for creating fatty acids
D) building blocks for creating carbohydrates

Answers
Answer:
B
Explanation:
building blocks for creating proteins
Why do you think the placebo effect works
Answers
Answer:
The placebo effect is when an improvement of symptoms is observed, despite using a nonactive treatment. It’s believed to occur due to psychological factors like expectations or classical conditioning.
Research has found that the placebo effect can ease things like pain, fatigue, or depression.
hope this will help u :)
which degrees of freedom are expected to contribute to the internal energy of a gas phase diatomic molecule at 298 k?
Answers
At high temperatures, a diatomic molecule possesses a total of six degrees of freedom.
Because a gas molecule may travel in any direction, it has three translational degrees of freedom. This is true for all gas molecules, whether monatomic, diatomic, or polyatomic, since every molecule in three-dimensional space may travel freely in all directions.
As a result, a diatomic molecule possesses five degrees of freedom: three translational and two translational.
As a result, a diatomic gas molecule possesses 6 degrees of freedom. This set may be divided into molecular translations, rotations, and vibrations. Three degrees of freedom are accounted for by the whole molecule's center of mass motion.
Learn more about diatomic molecule
https://brainly.com/question/11914539
#SPJ4
What is heterogenous mixture
Answers
Answer:
A heterogeneous mixture is simply any mixture that is not uniform in composition - it's a non-uniform mixture of smaller constituent parts. By contrast, a mixture that is uniform in composition is a homogeneous mixture.
A dog barks to alert his owner. What is the medium for the sound waves produced
by the dog barking? lesson 4.01
A. Air
B. ground
C. water
D. the ocean
Answers
Answer:
The Answer is ground or air
Is a long-coiled tube where most of the digestion takes place
Answers
Answer:
I'm guessing the alimentary canal
help!!!!! please
can someone do this i have NO clue
Answers
Mixture- honey, seawater, blood, mud
element- hydrogen, calcium
compund- magnesium oxide, copperII sulfate, potassium iodide solution.
What do you mean by mixture, element and compunds?A mixture is a material composed of two or more different chemical substances that are not chemically bonded. A mixture is the physical combination of two or more substances that retain their identities and are mixed in the form of solutions, suspensions, and colloids.
Element is a free and open-source instant messaging client that uses the Matrix protocol. End-to-end encryption, private and public groups, file sharing between users, voice and video calls, and other collaborative features are supported by Element via bots and widgets.
A compound is a substance made up of two or more different chemical elements combined in a fixed ratio in chemistry. When the elements combine, they react and form chemical bonds that are difficult to break. These bonds form as a result of atoms sharing or exchanging electrons.
To know more about element visit:
https://brainly.com/question/13025901
#SPJ1
Select the answer that best describes an aqueous solution made from each of the following substances:
solid sodium carbonate (Na 2CO 3)
acidic
basic
neutral
cannot tell
none of these (A-D)
Answers
An aqueous solution made from solid sodium carbonate (Na2CO3) would be basic.
The best description for an aqueous solution made from solid sodium carbonate (Na2CO3) is:
Your answer: basic
Sodium carbonate is a salt of a strong base (sodium hydroxide) and a weak acid (carbonic acid). When it dissolves in water, it undergoes hydrolysis and forms sodium hydroxide (NaOH) and carbonic acid (H2CO3). Since sodium hydroxide is a strong base and carbonic acid is a weak acid, the resulting solution will be more basic than acidic, making the aqueous solution basic.
To know about basic visit:
https://brainly.com/question/15347758
#SPJ11
The average volume of the acid was mixed with 25cm3 of pottasium carbonate solution to obtain potassium chloride solution.
Describe how you would obtain crystals of pottasium chloride from the solution.
Answers
Answer:
To obtain crystals of potassium chloride from the solution, you can follow the following steps:
1. Heat the solution to evaporate some of the water and concentrate the solution.
2. Continue heating the solution until crystals start to form at the bottom of the container.
3. Remove the container from heat and allow it to cool down to room temperature.
4. Once the solution has cooled, you can use a filter paper and funnel to filter out the crystals from the remaining solution.
5. Wash the crystals with a small amount of cold distilled water to remove any impurities.
6. Dry the crystals by placing them on a watch glass or filter paper in a warm place until all the water has evaporated.
By following these steps, you should be able to obtain pure crystals of potassium chloride from the solution.
What is the mass percent of a sugar solution having 15 g sugar dissolved in 63 g of solution?
Answers
Answer:
23.81%
Explanation:
The mass percent of a solution, also known as percentage by weight or w/w%, can be calculated using the formula:
mass percent = (mass of solute / mass of solution) x 100%
In this question;
Mass of solute (sugar) = 15g
Mass of solution (sugar + water) = 63g
Hence,
w/w% = 15/63 × 100
w/w% = 0.2381 × 100
w/w% = 23.81%
Therefore, the mass percent of the sugar solution is 23.81%.
What is soil conservation and water conservation?
Answers
Soil and water conservation are those activities at the local level which maintain or enhance the productive capacity of the land including soil, water and vegetation in areas prone to degradation. prevention or reduction of soil erosion, compaction, salinity; conservation or drainage of water and.
What do you mean by conservation of water?
Water conservation includes all policies, strategies and activities for the sustainable management of natural freshwater resources, for the protection of the hydrosphere and for meeting current and future human demand. The amount of water used affects population, household size, growth and well-being.To know more about conservation of water, click the link given below:
https://brainly.com/question/10929764
#SPJ4
How many moles are in 5.12 × 10³ F atoms?
Answer in units of mole
Answers
The number of moles in 5.12 × 10³ Fluorine(F) atoms is 8.45 × 10 ‐ ²³ moles.
The moles is defined as the measurement of the amount of any substance. Its is the measure of the amount of elementary particles in a substance. One mole is numerically equal to 6.023 × 10²³ which is called Avogadro's number.
From Periodic Table, we can find that Molecular Weight of Fluorine(F) is 19. We know the Molar Weight of any element is numerically equal to its Molecular Weight i.e. numerically, Molecular Weight = Molar Weight of any matter.
The number of moles in 5.12 × 10³ Fluorine(F) atoms will be calculated as: (5.12 × 10³) / (6.023 × 10²³) = 8.45 × 10 ‐ ²³ moles
So, there are approximately 8.45 x 10^-23 moles in 5.12 × 10³ atoms of Fluorine.
Learn more about Molar Weight :
https://brainly.com/question/14865338
which property cohesion or adhesion causes surface tension in water
Answers
Answer:
cohesion
Explanation:
because because cohesion is the state where particles are closer to eacch other hence tthe. paarticles being closer resul to. a floating objct like a razoor too experience surface tension.
note
thi condition ccaan only be remove by a ssurrfacctaant
CH4 (g)+ 202(g)=CO2(g) + 2H2O(l)If 133.2 grams of CH4 react with excess O2 , how many grams of H2O result?
Answers
299.7g of H2O will be produced.
1st) With the balanced chemical equation and the molar mass of CH4 and H2O, we can calculate the amount of H2O that is produced by stoichiometry:
- CH4 molar mass: 16g/mol
- H2O molar mass: 18g/mol
According to the balanced equation, 16g of CH4 will produce 36g of H2O (2 x 18g).
2nd) Now, with a mathematical rule of three we can calculate the grams of water that will be produced from 133.2 grams of CH4:
\(\begin{gathered} 16gCH_4-36gH_2O \\ 133.2gCH_4-x=\frac{133.2gCH_4\cdot36gH_2O}{16gCH_4} \\ x=299.7gH_2O \end{gathered}\)Finally, 299.7g of H2O will be produced from 133.2 grams of CH4.
This gland of the endocrine and digestive systems produces insulin to help control the body's sugar levels and enzymes which aid in digestion.
Answers
Answer:
Pancreas.
Explanation:
Living systems are self-organized life forms and are known to be very much interactive with their surroundings or environment. Also, living systems are dependent on the flow of information, matter and energy at various levels.
Some examples of living systems in organisms are respiratory system, nervous system, digestive system, and circulatory system.
Additionally, living systems comprises of the following components; cells, organs, muscle, tissues, blood, etc.
An endocrine system refers to a series of ductless glands and organs responsible for the production and secretion of hormones that are used by the body for the performance of various functions such as metabolism, controlling growth, reproduction, mood, sleep, etc. These hormones are secreted directly into the circulatory system (blood) and then transported to the organs and tissues in the body.
Pancreas is one of the glands of the endocrine and digestive systems that produces insulin to help control the body's sugar levels and enzymes which aid in digestion.
Which of the following compounds has ionic bonds?
a. H2O
b. O2
c. Ne
d. CO
e. KBr
Answers
The compound that has ionic bonds is KBr (potassium bromide). Therefore the correct option is Option E.
Ionic bonds develop when two atoms with significantly differing electronegativities create a bond in which one atom (the metal) contributes electrons to the other atom (the non-metal). Potassium (K) is a metal in KBr, while bromine (Br) is a nonmetal. The electronegativity of K is low, whereas that of Br is high. When K and Br bond, K contributes its valence electron to Br, resulting in an ionic bond.
The other chemicals listed, on the other hand, have covalent bonding. When atoms with similar electronegativities share electrons in order to produce a more stable electron configuration, covalent bonds occur.
a. H2O has covalent bonds;
b. O2 contains covalent bonds; and
c. Ne is a noble gas that does not create bonds.
d. CO contains covalent bonds.
For such more question on ionic bonds:
https://brainly.com/question/977324
#SPJ4
the half-life of kr-92 is 1.84 seconds. if the original mass is 24 mg, how much remains after 7.36 seconds?
Answers
After 7.36 seconds, 1.5 mg of Kr-92 remains.
To calculate the remaining mass of Kr-92 after a certain time, we can use the concept of radioactive decay and the equation:
N(t) = N₀ * (1/2)^(t / T₁/₂)
Where:
N(t) is the remaining quantity at time t,
N₀ is the initial quantity,
t is the elapsed time,
T₁/₂ is the half-life of the radioactive substance.
Given:
Initial mass (N₀) = 24 mg
Half-life (T₁/₂) = 1.84 seconds
Elapsed time (t) = 7.36 seconds
Let's calculate the remaining mass of Kr-92 after 7.36 seconds:
N(t) = N₀ * (1/2)^(t / T₁/₂)
N(7.36) = 24 mg * (1/2)^(7.36 / 1.84)
N(7.36) = 24 mg * (1/2)^4
N(7.36) = 24 mg * 1/16
N(7.36) = 1.5 mg
To know more about Kr refer here
https://brainly.com/question/29755230#
#SPJ11
bismuth-210 is an isotope that radioactively decays by about 13% each day, meaning 13% of the remaining bismuth-210 transforms into another atom (polonium-210 in this case) each day. if you begin with 193 mg of bismuth-210, how much remains after 6 days?
Answers
After 6 days, approximately 56.5 mg of bismuth-210 remains.
To learn more about bismuth here:
https://brainly.com/question/13721046
#SPJ11
The %Error of your experimentally determined density of a metal cylinder was determined in Part B Which measured quantity, the mass or the volume, is the greater source of error in your experimentally determined density of your metal cylinder? Explain why this quantity is the greater source of error. (2p 2. A perfect cube of aluminum metal has a mass of 20.00 grams. The density of aluminum is 2.70 g/cm3. Determine the length of one edge of the aluminum cube. (2 pts) 3. The fluid used in a car radiator is a mixture of water and ethylene glycol. A 15.00 mL volume of radiator fluid taken from a car has a mass of 15.69 g. a. Determine the density of the radiator fluid solution. (2 pts) b. Water can be assumed to have a density of 1.0 g/mL. Based on density of the radiator fluid solution, is the density of pure ethylene glycol is greater than or less than the density of pure water? Explain your reasoning for your answer.
Answers
In determining the greater source of error in the experimentally determined density of the metal cylinder, we need to consider the mass and the volume.
The mass and volume are both measured quantities, and any errors in these measurements can contribute to the overall error in the density calculation. However, in this case, the greater source of error is likely the volume measurement.
The volume of the metal cylinder is determined by measuring its dimensions, such as length, width, and height. These measurements are subject to uncertainties due to various factors, such as parallax errors or limitations of the measuring instrument. Even small errors in the measurements of length, width, or height can result in significant differences in volume when multiplied together.
On the other hand, the mass measurement of the metal cylinder is typically more precise and less prone to errors. Using a balance with a high level of accuracy, the mass can be measured directly without much uncertainty.
To minimize the error in the experimentally determined density, it is crucial to ensure accurate measurements of the volume. This can be achieved by using precise measuring instruments, taking multiple measurements, and averaging the results. Additionally, techniques like using a water displacement method can provide a more accurate volume measurement.
In conclusion, while both the mass and volume measurements contribute to the overall error in the experimentally determined density, the volume measurement is likely the greater source of error due to the inherent uncertainties in measuring dimensions accurately.
To know more about determining visit:
https://brainly.com/question/29898039
#SPJ11