As of 2017, the tallest roller coaster in the world is the Kingda Ka, located at Six Flags Great Adventure in Jackson, New Jersey. It stands 139 meters tall and, at one point, the cars drop 127 meters! Roller coasters are a great way to investigate energy. Look at the diagram of a roller coaster below, and answer the following questions. At what point on the track does the car have the greatest potential energy?
Answers
Answer:
energy due to its velocity. We can compare potential and kinetic energy by considering a car on a hill. When the car is at the top of the hill it has the most potential energy.
Related Questions
he number-average molecular weight of a polypropylene is 1,000,000 g/mol. Compute the degree of polymerization.
Answers
Answer:
The answer is "23765.4"
Explanation:
Motor weight average number \((\bar{M_n}) = 1000000 \frac{g}{mol}\)
Poly condensation degree dependent on the average number of molecular weights is as follows:
\(DP_n = \frac{\text{Mol.Wt Number Medium}}{\text{Monomer Unit Mol.Wt}}\)
All monomer module, in this case, is propylene
Sunrise. Unit Wt = Mol. Propylene weight
\(= \ Mol. \ Wt \ of \ C_3H_6\\\\= 3 \times 12.01 +6 \times 1.008 \ \ \frac{g}{mol}\\\\= 42.078 \frac{g}{mol}\\\\\)
\(DP_n = \frac{1000000}{42.078}\\\\\)
\(=23765.4\)
A certain bimolecular reaction at 40 °C at an activation energy of 30 kJ/mol. The addition of a catalyst reduces the activation energy by a factor of 2. How much faster does the catalyzed occur?
Select one:
OA. 318.63
OB. 358.63
C. 338.63
OD. 378.63
Answers
k = Ae^(-Ea/RT)
where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant (8.314 J/mol·K), and T is the temperature in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin:
40°C + 273.15 = 313.15 K
Now, let's find the ratio of the rate constants for the catalyzed and uncatalyzed reactions:
k_catalyzed / k_uncatalyzed = e^((Ea_uncatalyzed - Ea_catalyzed) / RT)
Since the catalyst reduces the activation energy by a factor of 2:
Ea_catalyzed = 30 kJ/mol / 2 = 15 kJ/mol
Convert the activation energies to J/mol:
Ea_uncatalyzed = 30,000 J/mol
Ea_catalyzed = 15,000 J/mol
Now, plug in the values:
k_catalyzed / k_uncatalyzed = e^((30,000 J/mol - 15,000 J/mol) / (8.314 J/mol·K × 313.15 K))
k_catalyzed / k_uncatalyzed ≈ 358.63
The catalyzed reaction occurs approximately 358.63 times faster. So, the correct answer is OB. 358.63.
The acid digestion test left 2.595 g of fiber from a composite specimen weighing 3.697 g. The composite specimen weighs 1.636 g in water. If the specific gravity of the fiber and matrix is 2.5 and 1.2, respectively, find the 1. Theoretical volume fraction of fiber and matrix 2. Theoretical density of composite
Answers
Answer:
0.4323 ; 1.5394
Explanation:
Given that :
Specific gravity of Fibre (Sf) = 2.5
Specific gravity of Matrix (Sm) = 1.2
Mass of Fibre, Mf = 2.595g
Weight of composite specimen in water , Wc = 1.636g
Composite weight, Ww = 3.697 g
Weight of Fibre and matrix
Volume fraction of Fibre and matrix :
[(Mf/Sf) ÷ (Mf/Sf + Wc/Sm)] * 100
[(2.595/2.5) ÷ (2.595/2.5 + 1.636/1.2)]
(1.038 ÷ 2.4013333)
= 0.43225986
= 0.4323
2.)
Theoretical density of composite :
Weight of composite / (Mf/Sf + Wc/Sm)
3.697 / (2.595/2.5 + 1.636/1.2)
3.697 / 2.4013333
= 1.5395613
= 1.5394
Consider Zn + 2HCl → ZnCl2 + H2 (g). If 0.30 mol Zn is added to HCl, how many mol H2 are produced?
Answers
Answer:
0.3 mol
Explanation:
Assuming HCl is in excess and Zn is the limiting reagent,
from the balanced equation, we can see the mole ratio of Zn:H2 = 1:1,
which means, each mole of zinc reacted gives 1 mole of H2.
So, if 0.30 mol Zn is added, the no. of moles of H2 produced will also be 0.3 mol, since the ratio is 1:1.
What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Hg2 concentration is 7.36E-4 M and the Al3 concentration is 1.05 M
Answers
Answer:
Explanation:
Concentration of Hg⁺² = 7.36 x 10⁻⁴ M
Concentration of Al⁺³ = 1.05 M
2Al + 3Hg⁺² = 2Al⁺³ + 3Hg .
E = E₀ + RT / nF ln [ Al⁺³]² / [ Hg⁺² ]³
E₀ = reduction potential of Hg⁺² minus reduction potential of Al⁺³
= 0.92 V - ( - 1.66 V )
= 2.58 V
E = 2.58 + .059 /n log [ Al⁺³]² / [ Hg⁺² ]³
n = 6 , [Al⁺³] = 1.05 M ; [Hg⁺²] = 7.36 x 10⁻⁴ M
E = 2.58 + .059 /6 log [ 1.05]² / [ 7.36 x 10⁻⁴ ]³
= 2.58 + .059 /6 log 27.65 x 10⁸ .
= 2.58 + .059 /6 [8+ log 27.65 ].
= 2.58 + .059 /6 [8+ log 27.65 ].
= 2.58 + .09
= 2.67 V .
how much energy is required to vaporize 2 kg of copper
Answers
It would require approximately 600 kilojoules of energy to vaporize 2 kg of copper.
To calculate the energy required to vaporize a substance, we need to consider the heat of vaporization, which is the amount of energy required to convert a given amount of substance from its liquid state to its gaseous state at a constant temperature.
The heat of vaporization for copper is approximately 300 kJ/kg (kilojoules per kilogram) at its boiling point, which is around 2567 degrees Celsius (4649 degrees Fahrenheit). This means that for every kilogram of copper, 300 kJ of energy is needed to vaporize it.
Given that you have 2 kg of copper, we can calculate the total energy required as follows:
Energy = Heat of Vaporization × Mass
Energy = 300 kJ/kg × 2 kg
Energy = 600 kJ
Therefore, it would require approximately 600 kilojoules of energy to vaporize 2 kg of copper.
It's worth noting that the heat of vaporization can vary slightly depending on the purity of the copper and the specific conditions, such as temperature and pressure. The value provided here is an approximation. Additionally, it's important to handle copper and any high-temperature processes with caution, as they can pose safety hazards.
for more questions on vaporize
https://brainly.com/question/24258
#SPJ8
Describes the chemical reaction (s) that produce AMD. Equations
are balanced and formatted to show subscripts.
Pls help I’m so confused
Answers
FeS2 + 7O2 + H2O → Fe2+ + 2SO4^2- + 2H+
This reaction is an oxidation reaction, where the sulfide mineral is oxidized to sulfate ions and ferrous ions are released. The ferrous ions can then react with water and oxygen to form ferric hydroxide (Fe(OH)3), which is a yellow-orange solid that contributes to the characteristic color of AMD.
The overall reaction can be written as:
4FeS2 + 15O2 + 14H2O → 4Fe(OH)3 + 8SO4^2- + 16H+
This reaction shows that four molecules of pyrite react with 15 molecules of oxygen and 14 molecules of water to produce four molecules of ferric hydroxide, eight molecules of sulfate ions, and 16 molecules of hydrogen ions. The reaction is balanced to ensure that the number of atoms of each element is the same on both sides of the equation.
The question is: What are the formulas for Ions and Compunds formed?
Ca, CL
Will mark Brainliest

Answers
Answer:
Ionic compounds are formed when positive and negative ions share electrons and form ionic bonds. The strong attraction between positive and negative ions often produces crystalline solids with high melting points. When there is a large difference in electronegativity between ions, ionic bonds are formed rather than covalent bonds. Cations, called cations, are listed first in the ionic compound formula, followed by negative ions, called anions.
Stable ionic compounds are electrically neutral in which electrons are shared between cations and anions to complete the outer electron shell or octant. When the positive and negative charges on the ions are the same or "cancel each other", you know you have the right formula for the ionic compound. Here are the steps to write and balance the formula: Identify the cation (the positively charged part). It is the weakest (most positively charged) ion. Cations include metals, which are usually on the left side of the periodic table. Identify anions (negatively charged moieties). It is the most negatively charged ion. Anions include halogens and nonmetals. Remember that hydrogen can carry a positive or negative charge in any way. Write the cation first, then the anion. Adjust the subscripts of the cations and anions so that the net charge is 0. Use the smallest integer ratio between the cations and anions to write the formula to balance the charges. If the cations and anions are of equal charge (eg +1/-1, +2/-2, +3/-3), then combine the cations and anions in a 1:1 ratio. An example is potassium chloride KCl. Potassium (K+) has a 1-charge, while chlorine (Cl-) has a 1-charge. Note that you don't write a subscript of 1. If the charges of the cation and anion are not equal, add subscripts to the ions as needed to balance the charges. The total charge of each ion is the subscript times the charge. Adjusted subscripts to balance charges. An example is sodium carbonate Na 2 CO 3 . The sodium ion has a +1 charge, multiplied by the subscript 2 gives a total charge of 2+. The carbonate anion (CO 3 -2 ) has a 2-charge, so there is no additional subscript. If you need to add a subscript to a polyatomic ion, enclose it in parentheses to clearly apply the subscript to the entire ion rather than individual atoms. An example is aluminum sulfate, Al 2 (SO 4 ) 3 . The parentheses surrounding the sulfate anion indicate that three 2-sulfate ions are required to balance 2 of the 3 charged aluminum cations.
Examples of ionic compounds
Many familiar chemicals are ionic compounds. A metal bonded to a non-metal is the death giveaway of an ionic compound you are dealing with. Examples include salts such as table salt (sodium chloride or NaCl) and copper sulfate (CuSO4).
How do people get minerals out of the ground?
Answers
Answer:
People get minerals out of the ground by digging it. They use machines to also help them dig faster.
Answer:
The primary method used to extract minerals from the ground are:
Underground mining, surface (open pit) mining. Placer mining...
Please mark me as a brainlist
How much energy is required to vaporize 1.5 kg of aluminum? (Refer to table
of latent heat values.)
A. 733 kJ
B. 1650 kJ
C. 7095 kJ
D. 600 kJ
Answers
Answer:
B 1650
Explanation:
A liquid has a mass of 3.35 g and volume of 5 mL. What is its density?*
D =
mass
volume
Answers
Explanation:
density= mass × volume
3.35×5
=. 16 .75
A student reacts a solution of vinegar (clear liquid; CH3COOH) with baking soda (white powder; NaHCO3). When the reaction is finished, there is white powder at the bottom of the reaction vessel. What is most likely the limiting reactant for this reaction, according to the equation below?CH3COOH + NaHCO3 --> NaCH3COO + H2O + CO2A.) NaHCO3 (baking soda)B.) NaCH3COO (sodium acetate)C.) CH3COOH (vinegar)D.) H2O (water)E.) CO2 (carbon dioxide)
Answers
According to our question, we have a white powder at the end of this reaction.
The white powder responds to the baking soda (NaHCO3)
We can exclude from our options, B, D, E because they are products, not reactants.
Thus, we have A and C.
One part of our text says: "When the reaction is finished, there is white powder at the bottom". This power is the NaHCO3, and it means that when the reaction finishes, this compound remains. Therefore is the excess.
So, our limiting reactant is C.) CH3COOH (vinegar)
Answer: C.) CH3COOH (vinegar)
If 3.05 mol of an ideal gas at 273 K has a volume of 208 L, what will its pressure be in kPa?
Use one of the following values:
R=0.0821 atm. L/mol. K
R= 8.31 kPa L/mol K
R= 62.4 torr. L/mol K
kPa
Answers
Ideal gas equation PV =nRT
The parameters are given in problem : moles (n) = 2.55 Volume (V) = 205L:Temperature(T) = 273K Pressure = ?kPa
Universal Gas constant R = 8.31kPa.L/mol.K substitue all these values in ideal gas equation in PV = nRT
PV = nRT
Px205 = (2.55)x(8.31)x(273)
P = (3.05)x(8.31)x(273) /205 kPa
P = 33.75 kPa.
The volume of gas particles is negligible. Gas particles are the same size and have no intermolecular forces as other gas particles. Gas particles move randomly according to Newton's laws of motion. An ideal gas is one that obeys the law pv=RT at all pressures and temperatures. At high temperatures and low pressure, the potential energy due to intermolecular forces becomes almost insignificant compared to the kinetic energy of the particles, and the size of the molecules increases compared to the intermolecular voids.
Learn more about An ideal gas here:-https://brainly.com/question/14812505
#SPJ1
what element is present in all organic compounds
Answers
Answer:
Explanation:
Except on Star Trek (the original there was a creature made of Silicon as a base) every organic compound has Carbon in it and usually hydrogen (but there are exceptions).
what would the ph be at the end of an enzyme-catalyzed reaction if it were carried out in a 0.1 m phosphate buffer of pka 6.86 and 0.005 m of acid was produced during the reaction
Answers
Answer:
Ph of the enzyme -catalyzed reaction = 6.77
Explanation:
Given data:
concentration phosphate buffer = 0.1 m
pka. = 6.86
concentration of acid in the reaction = 0.005 m
Determine the Ph at the end of the reaction
For a balanced buffer solution ; Acid amount = base amount
first we will determine the concentration of acid and base in relation to the buffer
acid = 0.1 / 2 = 0.05
base = 0.1 /2 = 0.05
The chemical reaction of the buffer can be expressed as attached below
PH = pka + log \((\frac{0.045}{0.055} )\) =
= 6.86 + log ( 0.8181 ) = 6.77

Which of the following is a solution?
A. Salt water
B. Macaroni and cheese
C. Cake mix
D. Substance floats in water
Answers
Answer:
salt water or cake mix but, I'm pretty sure salt water
Answer:
it is sugar i got it right on study island
Explanation:
Write the correct symbols or formula each of the following
1 one atom of oxygen
Answers
Answer:
The correct symbol for 1 atom of oxygen is O
Explanation:
The number of atoms of any element in the given chemical formula is the number that is written on the foot of the symbol of that element. The the correct symbols or formula of 1 one atom of oxygen is O.
What is atom?Atom is the smallest particle of any matter. Atom combines to form element and element combine to form molecule or compound.
Atom consists of electron, proton and neutron. The total mass of atom is inside the nucleus. Inside the nucleus proton and neutron is there. So calculate mass of an atom, total mass of all protons is added to the total mass of neutron. Electrons revolve around the nucleus.
Atom of any element is just the symbol of that element. The the correct symbols or formula of 1 one atom of oxygen is O.
Thus, the correct symbols or formula of 1 one atom of oxygen is O.
Learn more about atoms, here:
https://brainly.com/question/13518322
#SPJ2
hi lovessssssss
Hsnsgehsndgd
Answers
If I have 340 mL of a 0.5 M NaBr solution, what will the concentration be if I add 560 mL more water to it?
Answers
Answer:
The correct answer is "0.31 M".
Explanation:
Given,
Volume,
\(V_1=340\)
\(V_2=560\)
Molarity,
\(M_1=0.5\)
Let the M₂ be "x".
By using the dilution formula, we get
⇒ \(M_1V_1=M_2V_2\)
then,
⇒ \(0.5\times 340=x\times 560\)
⇒ \(170=x\times 560\)
⇒ \(x=\frac{170}{560}\)
⇒ \(=0.31 \ M\)
What are aliphatic aldehydes? Class 12
Answers
Answer:
Explanation: The aldehydes in which the aldehydic functional group (−CHO) is attached to a saturated carbon chain are called Aliphatic aldehydes.
WILL GIVE 50 POINTS AND BRAINLIEST
Plate Tectonics Lab Report
Instructions: In the Plate Tectonics lab you will investigate the interactions between continental and oceanic plates at convergent, divergent, and transform boundaries around the globe. Record your observations in the lab report below. You will submit your completed report.
Name and Title:
Include your name, instructor's name, date, and name of lab.
Objective(s):
In your own words, what was the purpose of this lab?
Hypothesis:
In this section, please include the if/then statements you developed during your lab activity for each location on the map. These statements reflect your predicted outcomes for the experiment.
Location One: Select two events that you predict will be observed. If I explore two continental plates at a convergent boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Two: Select three events that you predict will be observed. If I explore two continental plates at a divergent boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Three: Select three events that you predict will be observed. If I explore two continental plates at a transform boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Four: Select two events that you predict will be observed. If I explore two oceanic plates at a convergent boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Five: Select three events that you predict will be observed. If I explore two oceanic plates at a divergent boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Location Six: Select two events that you predict will be observed. If I explore two oceanic plates at a transform boundary, then I will observe:
earthquakes
faults
ocean formation
mountains
volcanoes
island chains
seafloor spreading
Procedure:
The procedures are listed in your virtual lab. You do not need to repeat them here. Please be sure to identify the test variable (independent variable), outcome variable (dependent variable).
Reminder: Test variable = the item you are changing or manipulating; Outcome variable = the item you are measuring
Test variable (independent variable):
Outcome variable (dependent variable):
Data:
Record the data from each location below.
Location Name Boundary Type
(C=Convergent, D=Divergent, or T=Transform) Year Observed
(5, 10, or 20 million years) Geologic Events Observed
(earthquakes, faults, ocean formation, mountains, volcanoes, island chains, seafloor spreading)
Location One
Himalayas 5 Event 1-
20 Event 2-
Location Two
East Africa 5 Event 1-
10 Event 2-
20 Event 3-
Location Three
San Andreas fault zone 5 Event 1-
10 Event 2-
20 Event 3-
Location Four
Aleutian Islands 5 Event 1-
20 Event 2-
Location Five
Mid-Atlantic Ridge 5 Event 1-
10 Event 2-
20 Event 3-
Location Six
Alpine Fault 5 Event 1-
20 Event 2-
Conclusion:
Your conclusion will include a summary of the lab results and an interpretation of the results. Please write in complete sentences.
What types of geological events or changes occur at divergent plate boundaries?
What types of geological events or changes occur at convergent plate boundaries?
What types of geological events or changes occur at transform plate boundaries?
Explain how these geological processes and interactions have changed Earth's surface through the years. Be sure to use evidence to support your answer.
Answers
Answer:
here are what i have so far, im doing this right now
Explanation:
Plate Tectonics Lab Report
Instructions: In the Plate Tectonic lab you will investigate the interactions between continental and oceanic plates at convergent, divergent, and transform boundaries around the globe. Record your observations in the lab report below. Y
Objective(s):
To look at interactions between continental and oceanic plates, etc.
Hypothesis:
In this section, please include the if/then statements you developed during your lab activity for each location on the map. These statements reflect your predicted outcomes for the experiment.
Location One: Select three events that you predict will be observed. If I explore two continental plates at a convergent boundary, then I will observe:
• earthquakes
• mountains
• volcanoes
Location Two: Select three events that you predict will be observed. If explore two continental plates at a divergent boundary, then I will observe:
• ocean formation
• volcanoes
• seafloor spreading
you will submit your completed report
ps; you might want to change up the objective.
A geological event is a brief, spatially diverse, dynamic and ongoing occurrence in the history of the Earth.
What is geological event?A geological event is a brief, spatially diverse, dynamic (diachronous), and ongoing occurrence in the history of the Earth that aids in the modification of the Earth system and the production of geological strata. The concept of event stratigraphy initially came up as a way to identify, analyse, and correlate how significant physical and biological events have affected the overall stratigraphical record.
Israel's Dead Sea basin Holocene sediments contain seismic activity. This can be considered a record of a geological event, an earthquake, that altered the strata. Geological events can occur over timescales of order of magnitude, from just a few seconds through millions of years, as well as on a variety of spatial scales, from the local to the globe.
1. Volcanoes and minor earthquakes
2. Volcanoes, earthquakes and fold mountains.
3. Earthquakes and fold mountains.
4. Magma from volcanoes is filled with nutrients that makes land fertile.
Therefore, a geological event is a brief, spatially diverse, dynamic and ongoing occurrence in the history of the Earth.
To know more about geological event, here:
https://brainly.com/question/2372671
#SPJ3
A steel bar and a copper bar have the same length of 1.500 m at -12.00 ∘C.
What is the difference in the lengths of the two bars at 41.0 ∘C ?
Answers
ΔL = αLΔT
where ΔL is the change in length, α is the coefficient of linear expansion, L is the initial length, and ΔT is the change in temperature.
The coefficient of linear expansion for steel is 1.2 x 10^-5 K^-1, and for copper is 1.7 x 10^-5 K^-1.
The change in temperature is:
ΔT = 41.0 - (-12.0) = 53.0 °C
The change in length for the steel bar is:
ΔL_steel = α_steel * L * ΔT = 1.2 x 10^-5 * 1.500 * 53.0 = 0.001914 m
The change in length for the copper bar is:
ΔL_copper = α_copper * L * ΔT = 1.7 x 10^-5 * 1.500 * 53.0 = 0.002565 m
Therefore, the difference in the lengths of the two bars at 41.0 °C is:
ΔL_copper - ΔL_steel = 0.002565 - 0.001914 = 0.000651 m.
,
I’m just gonna keep doing these keep answers ga me you will get brainliest
Answers
Answer:
Thx
Explanation:
A chemist has a small amount of compound that has the boiling point 65°c that must be fractionally distilled. Yet, the chemist doesn't want to lose any of the compound to hold up on the column. What the chemist should do?
Answers
Answer:
A "chaser," a high-boiling compound whose vapors will displace the vapors of the desired low-boiling compound, can be used to distill a small amount of compound.
A smaller fractionation apparatus or a Vigreux column could be used instead.
Explanation:
What are the products of a chemical reaction?
Answers
Answer: Chemical reactions occur when chemical bonds between atoms are formed or broken. The substances that go into a chemical reaction are called the reactants, and the substances produced at the end of the reaction are known as the products.
A dunk tank holds 550,200 grams of water. How many moles of water are in the tank?
Answers
Answer:
Aà bhûtÿ ñhjkjłfd hÿrèërqw
Explanation:
What does it mean for a reaction to release energy?
a. the relative potential energy of the reaction is negative
b. the activation energy of the reaction is positive.
c. The relative potential energy of the reaction is positive.
d. The activation energy of the reaction is negative
Answers
The activation energy of the reaction is positive when a reaction release energy.
Chemical reactions that release energy are called exothermic.
What is exothermic reaction.Exothermic reaction is the reaction that release energy during the formation of bonds in the products.
Energy is released when bonds are formed in products compare to the reactants.
Therefore, activation energy of the reaction is positive because it is the energy that is needed to break barriers for reaction to start and it release chemical energy during reaction.
For more details on Exothermic reaction, check the link below.
https://brainly.com/question/2924714
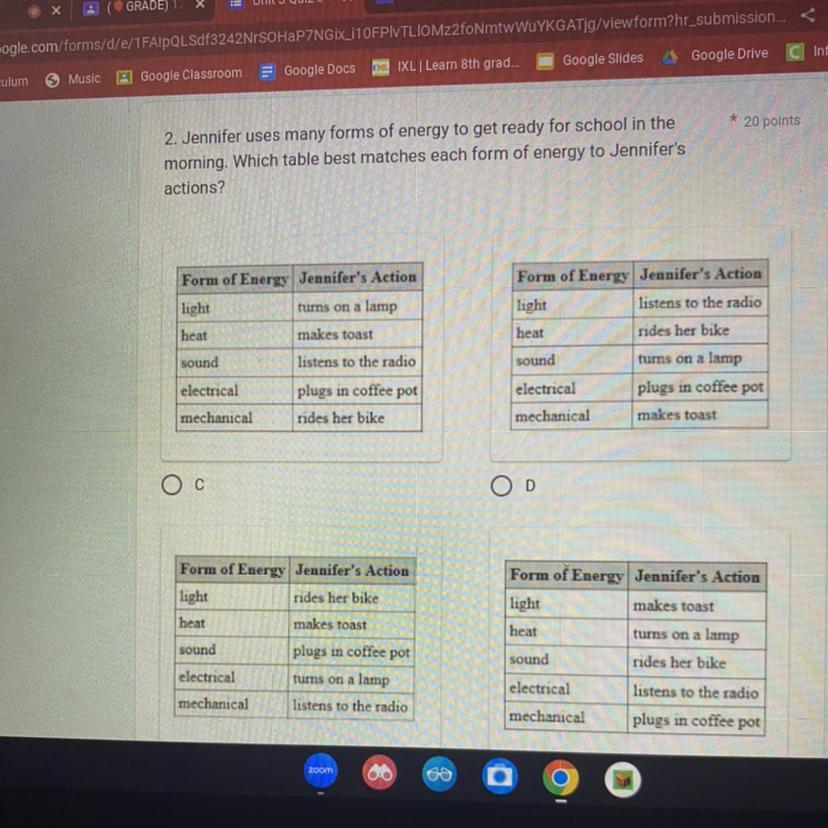
2. Jennifer uses many forms of energy to get ready for school in the
morning. Which table best matches each form of energy to Jennifer's
actions?
Form of Energy Jennifer's Action
light
turns on a lamp
makes toast
listens to the radio
plugs in coffee pot
rides her bike
JOSTAGENS
sound
O C
E
electrical
mechanical
Form of Energy Jennifer's Action
Light
rides her bike
makes toast
plugs in coffee pot
turns on a lamp
listens to the radio
sound
electrical
mechanical
S
Form of Energy Jennifer's Action
Light
listens to the radio
heat
rides her bike
sound
turns on a lamp
electrical
plugs in coffee pot
mechanical
makes toast
O D
* 20 points
Form of Energy Jennifer's Action
light
heat
sound
electrical
mechanical
makes toast
turns on a lamp
rides her bike
listens to the radio
plugs in coffee pot
Answers
The table best matches each form of energy to Jennifer's actions is A.
What is energy?Energy is defined as the capacity to labor long hours or be extremely busy without becoming exhausted. Red blood cells, which carry oxygen in the blood throughout the body, are created by vitamin B12 in the body. Your body's cells use the oxygen once it gets there to produce energy.
Electrical energy, chemical energy obtained from fuels, food, and energy derived from the sun are the major types of energy used in our homes. Everyday appliances convert electrical energy into a variety of forms, including mechanical/kinetic, sound, heat, light, and other types of electromagnetic radiation.
Thus, the table best matches each form of energy to Jennifer's actions is A.
To learn more about energy, refer to the link below:
https://brainly.com/question/1932868
#SPJ1
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
How many moles are there in 5.24 Liters of NH3 gas at STP?
Answers
Answer:
34.3
Explanation: