At 40.°C, the concentration of hydronium (H3O+) ions in an aqueous solution is 2.9 ✕ 10−5 M. (Note: At 40.°C, the ion-product constant for water is 2.87 ✕ 10−14.) (a) What is the concentration of hydroxide (OH −) ions in this solution?
Answers
To find the concentration of hydroxide (OH-) ions in the solution when the concentration of hydronium (H3O+) ions is 2.9 ✕ 10−5 M at 40°C, you'll need to use the ion product constant of water (Kw).
Step 1: Recall the ion product constant of water (Kw) expression:
Kw = [H3O+] × [OH-]
Step 2: At 40°C, the value of Kw is approximately 2.92 × 10^-14.
Step 3: Use the given concentration of hydronium ions ([H3O+]) and plug it into the Kw expression:
2.92 × 10^-14 = (2.9 × 10^-5) × [OH-]
Step 4: Solve for the concentration of hydroxide ions ([OH-]):
[OH-] = (2.92 × 10^-14) / (2.9 × 10^-5)
Step 5: Calculate the result:
[OH-] ≈ 1.01 × 10^-9 M
The concentration of hydroxide (OH-) ions in the solution is approximately 1.01 × 10^-9 M.
To know more about hydroxide and hydronium :
https://brainly.com/question/2817451
#SPJ11
Related Questions
How many liters of air must react with 2500 mL of hexane in order for combustion to occur completely. The percentage of oxygen in air is 20.9%.
Answers
Answer:
118750 ml
Explanation:
The chemical equation for complete combustion of hexane is given as;
2C6H14 + 19O2 → 12CO2 + 14H2O
From the equation of the reaction;
2 mol of C6H14 reacts with 19 mol of O2
2 ml of C6H14 reacts with 19 ml of O2
2500 mL of C6H14 would react with x ml of O2
2 = 19
2500 = x
x = 2500 * 19 / 2 = 23750 ml
Since oxygen is 20% of air;
23750 = 20 / 100 * (Volume of air)
Volume of air = 23750 * 100 / 20 = 118750 ml
The literature value for the melting point of your product was 154-155 °C. Below is the data for 4 students, which student had the purest crystals AND the correct product. Student A: melting point range of 141-147 °C Student B: melting point range of 158-159 °C Student C: melting point range of 145-155 °C Student D: melting point range of 151-152 °C B с D A
Answers
Therefore, Student C had the purest crystals and the correct product
Melting point determination is a useful technique for checking the purity and identity of the crystalline solid. The melting point of a compound is the temperature range where the solid changes to a liquid. The literature value for the melting point of a product is an important property to know, and it can be compared to the values obtained experimentally.
The melting point of the compound is an important factor in determining its purity. A pure compound has a sharp melting point, whereas an impure compound has a broad melting range that is lower than the pure melting point.
The student with the purest crystals and the correct product was Student C.
Student A's melting point range is too low, which indicates that the compound is impure.
The melting point range of Student B is too high, which indicates that the compound is impure.
The melting point range of Student D is close to the literature value, but it is still too broad, which indicates that the compound is impure.
Student C's melting point range is close to the literature value, and it is relatively sharp, which indicates that the compound is pure.
to know more about melting point visit:
https://brainly.com/question/31109629
#SPJ11
If a number has the units g/L then its
A) a number we are not going to use at all.
B) an imaginary unit that we are not going to use.
C) creating a relationship between mass and volume
D) a relationship between volume and distance.
Answers
Answer:
C) creating a relationship between mass and volume
g/L
g or gram is mass
L or Litres is for volume
()3C− − on reaction with HI gives () − − as
the main products and not () − and −
Answers
3C⁻⁻ on reaction with HI gives I⁻⁻⁻ as the main products and not H⁻ and C₂H₅I.
When 3C⁻⁻ is reacted with HI, the reaction product obtained is I⁻⁻⁻ as the main product. The C₂H₅I and H⁻ are not produced in significant quantities and cannot be considered the main product.The 3C⁻⁻ compound reacts with HI in the presence of a solvent to produce hydrogen gas, H⁻, C₂H₅I, and I⁻⁻⁻. The primary product obtained is I⁻⁻⁻ because it is stable and has a higher energy than C₂H₅I and H⁻.However, the reaction can be controlled to obtain C₂H₅I and H⁻ as the primary products by changing the reaction conditions. The reaction must be carried out in anhydrous conditions and at a low temperature so that the reaction proceeds in the desired direction.
3C⁻⁻ on reaction with HI gives I⁻⁻⁻ as the main products and not H⁻ and C₂H₅I. However, the reaction can be controlled to obtain C₂H₅I and H⁻ as the primary products by changing the reaction conditions.
To know more about hydrogen visit:
brainly.com/question/30623765
#SPJ11
19. Which of the following is NOT a function of proteins?
a. provides structural support for cells
b. long term energy storage
c. speeds up reactions
d. movement and transportation
Answers
g a rigid waled cubical container is completely filled with water at 40 f and sealed. the water is then heated to 100 f. determine the pressure that develops in the container when the water reaches this higher termperature
Answers
The pressure that is develops in the container when the water is reaches this higher temperature is 2.03 × 10³ psi.
According to the law of mass of conservation , wet :
Density (40 °C) × V = Density ( 100 °C ) × (V + ΔV)
ΔV/ V = ( Density (40 °C) / Density ( 100 °C ) ) - 1
ΔV/ V = 1.940 / 1.927
ΔV/ V = 0.00675
The change in volume and bulk modulus relation given as :
K = - ΔP / ( ΔV/ V)
ΔP = - 300000 × - 0.00675
ΔP = 2.03 × 10³ psi
To learn more about bulk modulus here
https://brainly.com/question/14602128
#SPJ4
A model of Nuclear Fusion?
Answers
Answer:
a proton turns into an atom that goes kabloey into fission products and other neutrons.
Explanation:
chemical formula of magnesium nitrate
Answers
Hello.
Magnesium nitrate is a chemical compound with the formula Mg (NO₃) ₂. Magnesium nitrate is an inorganic salt very soluble in water and alcohol and has the appearance of fine white crystals.
Magnesium Nitrate is commonly used as a fertilizer due to its high nutritional value, since it provides Magnesium and Nitrogen, these are important nutrients in the growth of crops. It is a water soluble fertilizer. Contains 10.5% N - 15.6% MgO. Provides nitrogen in the form of nitrate.

"Which of the following reagents would oxidize Zn to Zn2+, but not Sn to Sn2+?Br2Br-Ca^2+Co^2+CaCo"
Answers
The only reagent that could potentially oxidize Zn to Zn²⁺ without oxidizing Sn to Sn²⁺ is Br₂ (bromine).
To determine which reagent would oxidize Zn to Zn²⁺ but not Sn to Sn²⁺, we need to compare the reduction potentials (E°) of the elements involved. The reagent with a higher reduction potential will have a greater tendency to accept electrons and oxidize the other element.
The reduction potential for Zn²⁺/Zn (Zn²⁺ + 2e⁻ ⇌ Zn) is approximately -0.76 V, while the reduction potential for Sn²⁺/Sn (Sn²⁺ + 2e⁻ ⇌ Sn) is approximately -0.14 V. Since the reduction potential for Zn²⁺/Zn is lower than that of Sn²⁺/Sn, Zn is less easily oxidized compared to Sn.
Now, let's examine the given reagents:
Br₂: Bromine (Br₂) has a higher reduction potential than Zn²⁺/Zn. It could potentially oxidize Zn to Zn²⁺. However, it can also oxidize Sn to Sn²⁺ because its reduction potential is higher than both Zn²⁺/Zn and Sn²⁺/Sn.
Br-: Bromide ion (Br-) has a lower reduction potential than both Zn²⁺/Zn and Sn²⁺/Sn. It would not oxidize either Zn or Sn.
Ca²⁺+: Calcium ion (Ca²⁺) has a lower reduction potential than both Zn²⁺/Zn and Sn2+/Sn. It would not oxidize either Zn or Sn.
Co²⁺: Cobalt(II) ion (Co²⁺) has a lower reduction potential than both Zn²⁺/Zn and Sn²⁺/Sn. It would not oxidize either Zn or Sn.
CaCo: This combination does not represent a known reagent or species and cannot be evaluated in terms of its oxidation potential.
Based on the given options, the only reagent that could potentially oxidize Zn to Zn²⁺ without oxidizing Sn to Sn²⁺ is Br₂ (bromine). However, it's important to note that in practical scenarios, multiple factors can influence redox reactions, so careful experimental considerations may be required to determine the actual outcome.
To know more about reduction potential, refer to the link below:
https://brainly.com/question/31362624#
#SPJ11
Which material would take the longest to cool off once heated?
Liquid water
Lead
Ice
Copper

Answers
Answer:
Liquid water
Bored pls talk to me.
my Roblox account name jxal1...........
Answers
Explanation:
Jesus Christ had dreads so shake em', I ain't got none but I'm planning on growing some
Answer:
ooo_SoSpooky add me :>
If a patient has a medical condition that causes his cells to absorb fewer than normal molecules, this patient would likely feel very tired. Explain your reasoning.
Answers
Answer:oxygen Explanation:The medical condition described here is anaemia. It is a blood cell disorder whereby the red blood cell doesn't function properly and hence doesn't carry enough oxygen to the tissues. This is usually caused when ones body is deficient of iron.The symptoms that may occur to such patients are weakness, fatigue, headache and pale skin.Based on the explanation, the answer is oxygen
Explanation:
A sample of hexane (C6H14) has a mass of 0.580 g. The sample is burned in a bomb calorimeter that has a mass of 1.900 kg and a specific heat of 3.21 J/giK. What amount of heat is produced during the combustion of hexane if the temperature of the calorimeter increases by 4.542 K? Use q equals m C subscript p Delta T..
Answers
The mass of the hexane sample is 0.580 g. The mass of the bomb calorimeter is 1.900 kg, and its specific heat is 3.21 J/g·K. The change in temperature is 4.542 K. So, heat produced during the combustion of hexane is 11195.1576 J.
To calculate the amount of heat produced during the combustion of hexane, we can use the formula q = m × C × ΔT.
First, we need to determine the mass of the hexane sample, which is given as 0.580 g.
Next, we need to find the mass of the bomb calorimeter, which is given as 1.900 kg.
Note that we need to convert the mass to grams by multiplying it by 1000, so the mass is 1900 g.
The specific heat of the bomb calorimeter is given as 3.21 J/g·K.
Finally, we have the change in temperature, which is given as 4.542 K.
Now, we can substitute these values into the formula: q = (0.580 g + 1900 g) × 3.21 J/g·K × 4.542 K.
Simplifying the equation, we get:
q = 2467.8 J/g·K × 4.542 K
Calculating the product:
q = 11195.1576 J
To know more about combustion refer to this:
https://brainly.com/question/31123826
#SPJ11
A candle produces a yellow flame on burning while LPG burning in a gas
burner produces a blue flame. Explain.
Answers
Due to Incomplete combustion, a candle produces a yellow flame and due to complete combustion, an LPG burner produces a blue flame.
A candle is made of wax, which does not burn completely. As a result, candles have partial combustion, which gives them their yellow color. On the other hand, LPG (Liquified Petroleum Gas), burns at approx. around 1,960°C temperature. Hence, LPG burners have complete combustion, giving them their blue color.
Read more about combustion,
https://brainly.com/question/23992512
what part of the human body provides milk
Answers
Answer: womans chest area
Explanation:
How many milliliters of 0. 250M NaOH is required to neutralize 30. 4mL of 0. 152M HCl?
Answers
Approximately 18.4832 mL of 0.250 M NaOH is required to neutralize 30.4 mL of 0.152 M HCl.
To determine the volume of 0.250 M NaOH required to neutralize 30.4 mL of 0.152 M HCl, we can use the concept of stoichiometry and the balanced chemical equation for the neutralization reaction between NaOH and HCl:
NaOH + HCl -> NaCl + H2O
From the balanced equation, we can see that the stoichiometric ratio between NaOH and HCl is 1:1. This means that for every mole of NaOH, we require an equal number of moles of HCl to neutralize.
First, let's calculate the number of moles of HCl present in the given volume:
Moles of HCl = concentration of HCl * volume of HCl
= 0.152 M * 30.4 mL
= 4.6208 mmol (millimoles)
Since the stoichiometric ratio is 1:1, the number of moles of NaOH required to neutralize the HCl is also 4.6208 mmol.
Now, let's calculate the volume of 0.250 M NaOH needed to contain 4.6208 mmol:
Volume of NaOH = (moles of NaOH) / (concentration of NaOH)
= 4.6208 mmol / 0.250 M
= 18.4832 mL
Therefore, approximately 18.4832 mL of 0.250 M NaOH is required to neutralize 30.4 mL of 0.152 M HCl.
learn more about NaOH here
https://brainly.com/question/20573731
#SPJ11
Calculate the pH of a 0.25 M solution of NaNO2 (Ka(HNO2) = 4.5 x 10^-4) (1.97)
a) pH = 3.35
b) pH = 4.45
c) pH = 5.55
d) pH = 6.65
Answers
The pH of a 0.25 M solution of NaNO2= 6.65.
Given the concentration of NaNO2, we can find the concentration of NaOH and HNO2 as follows:
NaNO2 = 0.25 MNaOH = HNO2 = x
(since they have equal concentrations due to the stoichiometry of the reaction)
Thus, we can write the equilibrium constant expression as:
Ka = x^2/0.25
Now, let's solve for x:
x^2 = 0.25 x 4.5 x 10^-4x = √(0.25 x 4.5 x 10^-4) = 0.015
This value represents the concentration of both HNO2 and NaOH. Since we are interested in pH, we need to find the concentration of H+ ions using the following equation:
Kw = [H+][OH-]
Since we have found the concentration of OH- (which is the same as the concentration of NaOH),
we can solve for H+:
Kw = 1.0 x 10^-14[H+][0.015] = 1.0 x 10^-14[H+] = 6.7 x 10^-13
Finally, we can find pH:
pH = -log[H+]pH = -log(6.7 x 10^-13)pH = 6.65
Therefore, the correct option is d) pH = 6.65.
learn more about pH here
https://brainly.com/question/172153
#SPJ11
what are 5 physical changes in matter
Answers
Answer:
cutting, bending, dissolving, freezing, and boiling
Explanation:
A physical change is a change in one or more physical properties of matter without any change in chemical properties. In other words, matter doesn't change into a different substance in a physical change. Examples of physical change include but are not limited to, from solid to liquid or from liquid to gas are also physical changes.
help please please help help help help help help help help!!!!!!!
how did chlorine atom attain saturation in potassium chloride and oxygen dichloride?
Answers
Answer:
because it was in the mixture
Explanation:
Some horses are bred for speed while other horses are bred for pulling heavy loads. What is the main influence on weather a horse will become a racehorse or farm horse?
Answers
Answer:
Good environmental and weather conditions.
Explanation:
The main influence for a horse that will become a racehorse or farm horse are good environmental and weather conditions. It also needs good food and proper training to made a racehorse. High quality race of a horse always prefer for making a racehorse because it has higher agility, speed, and spirit as compared to horse belongs to ordinary race.
Which area of biotechnology would most likely create ethical concerns? *
Answers
Answer:
organ cloning for use in transplants.
Explanation:
3. How is the metallic bonding different than ionic or covalent bonding? What are some properties of metals that
result from this type of bonding? Explain/connect how the nature of the bonding leads to the properties of
metallic substances.
Answers
Metallic bonding is different than ionic or covalent bond as that Metallic bond form when atoms share number of electrons in a metal lattice. The properties of metals that result from this type of bonding are: Malleability, Ductility, High melting point and boiling point. The nature of the bonding leads to the properties of metallic substances is that metallic bonds involves electrostatic forces of attraction.
What is Ionic Bond ?Ionic bonds is a chemical bond formed when one atom transfer its valence electron to another atom. Ionic bond is also called electrovalent bond. These bonds are stronger.
What is Covalent Bond ?A chemical bond in which pairs of electrons are shared between the two atoms is known as Covalent Bond. Covalent bond is also called molecular bond.
Metallic bonding is different than ionic or covalent bond as that Metallic bond form when atoms share number of electrons in a metal lattice while Covalent bond form when pairs of electrons are shared between the two atoms and Ionic bond form when one atom transfer its valence electron to another atom.
The properties of metals that result from this type of bonding are:
MalleabilityDuctilityHigh melting point and boiling pointMetallic LustreHigh electrical and thermal conductivityThe nature of the bonding leads to the properties of metallic substances is that metallic bonds involves electrostatic forces of attraction. The delocalized electrons are highly mobile due to the metallic bonds.
Thus from the above conclusion we can say that Metallic bonding is different than ionic or covalent bond as that Metallic bond form when atoms share number of electrons in a metal lattice. The properties of metals that result from this type of bonding are: Malleability, Ductility, High melting point and boiling point. The nature of the bonding leads to the properties of metallic substances is that metallic bonds involves electrostatic forces of attraction.
Learn more about the Ionic bond here: brainly.com/question/13526463
#SPJ1
Can anyone please answer questions 3 and 4
I’ll give the brainiest!
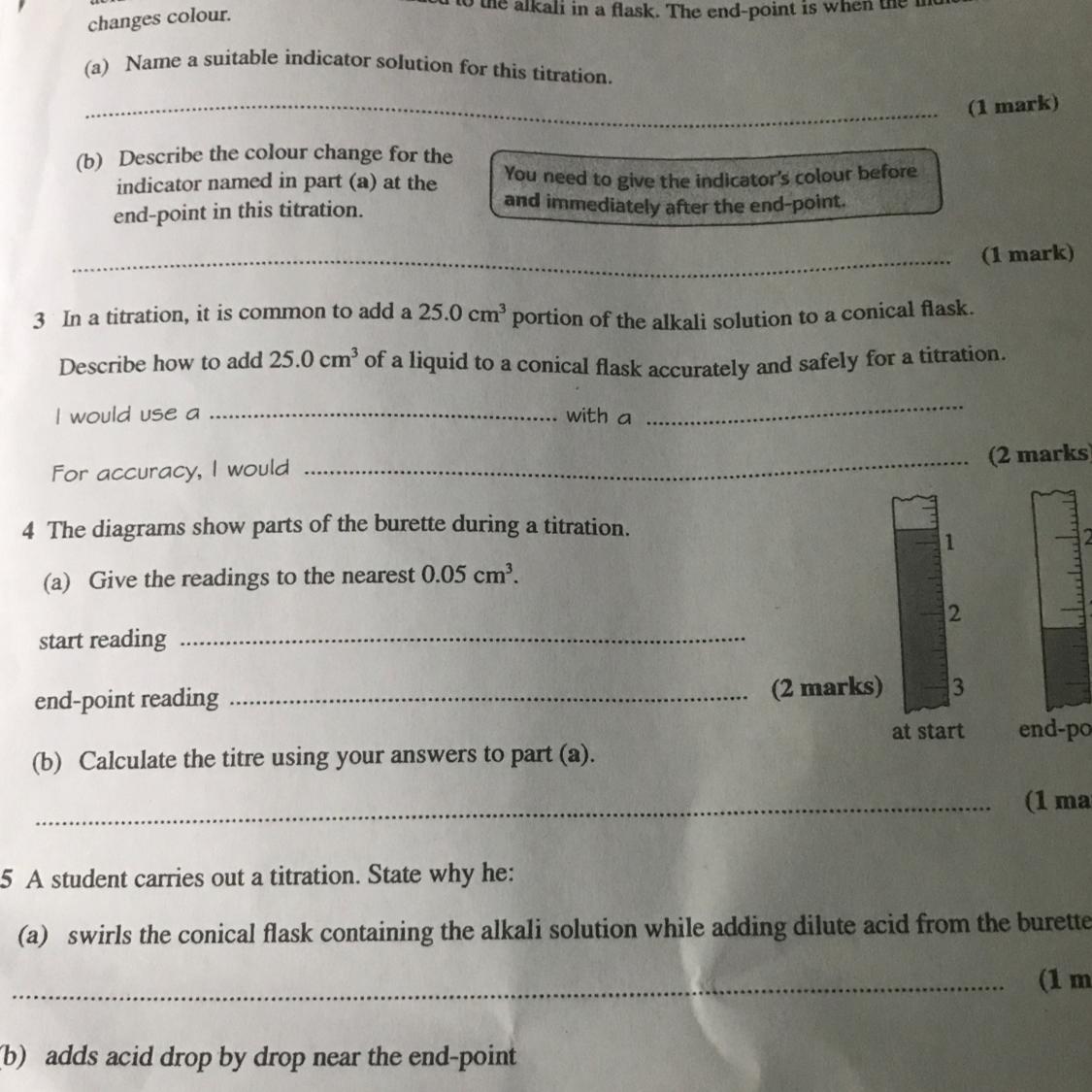
Answers
Answer:
1.Phenolphthalein
2.Method
a.Use the pipette and pipette filler to add 25 cm 3 of alkali to a clean conical flask.
b.Add a few drops of indicator and put the conical flask on a white tile.
c.Fill the burette with acid and note the starting volume.
d.Slowly add the acid from the burette to the alkali in the conical flask, swirling to mix.
Explanation:
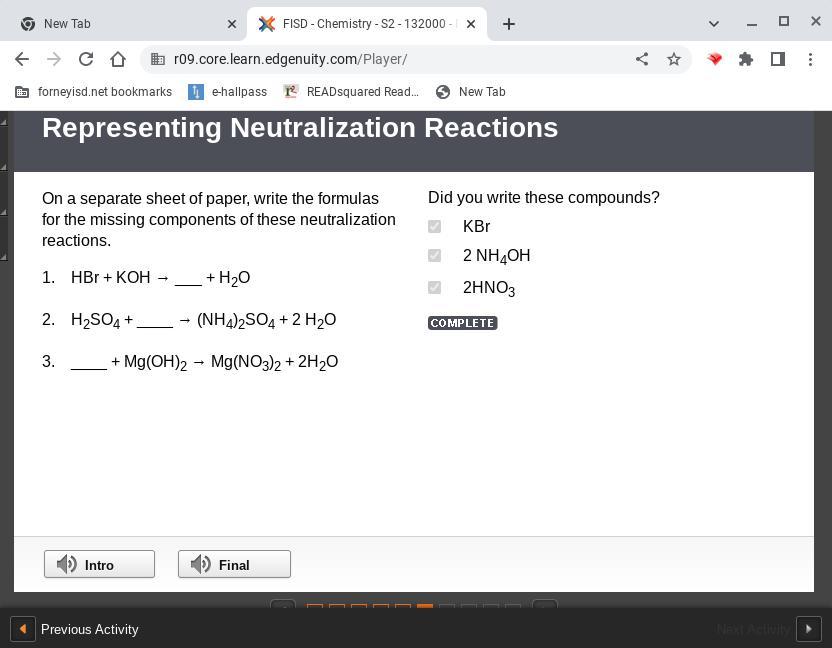
On a separate sheet of paper, write the formulas for the missing components of these neutralization reactions. 1. hbr koh → ___ h2o 2. h2so4 ____ → (nh4)2so4 2 h2o 3. ____ mg(oh)2 → mg(no3)2 2h2o
Answers
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
What is a neutralization reaction?A neutralization reaction is a reaction that occurs between an acid and a base to yield salt and water only.
The missing products of the neutralization reactions are;
1) KBr
2) NH3
3) HNO3
Learn more about neutralization:https://brainly.com/question/15395418
#SPJ4
Answer:
KBr
2 NH4OH
2 HNO3
Explanation:
How is a star first born
Answers
What state of matter is water at 80 degrees celsius?
Answers
Answer:
Liquid
Explanation:
Because water becomes a solid (freezes) at 0°C and becomes a gas (evaporates) at 100°C, it is liquid at any temperature in between.
Which species will have the strongest mass shift on a magnetic susceptibility balance?.
Answers
O2 is the correct answer.
Explanation:
On a magnetic susceptibility balance, the O2 species will have the strongest mass shift since stronger paramagnetic species will have a larger mass shift.
The oxygen atoms in the O2 species are paramagnetic because unpaired electrons rotate in the same direction, increasing the magnetic field force. As a result, the oxygen atoms with two unpaired electrons will exhibit the largest mass shift on a magnetic susceptibility balance.
The magnitude of the mass shift is -O2, which increases with species paramagneticity. The mass shift increases with species paramagneticity. Therefore, on a magnetic susceptibility balance, oxygen will have the highest mass shift since it has two unpaired electrons in the molecular orbital diagram.
To know more about magnetic susceptibility balance visit:-
https://brainly.com/question/2285863
#SPJ4
How much heat is required to melt 20 g of gold at 1064.18 °C with a heat of fusion of 64 J/g *
Answers
Answer:
1280J are required.
Explanation:
Heat of fusion is defined as the amount of heat required to change its state from liquid to solid at its melting point at constant pressure.
As heat of fusion of gold is 64J/g, there are required 64J to melt 1g of gold at its melting point. The energy required to melt 20g is:
20g * (64J/g) =
1280J are required
Calculate the % yield if 203 grams of CL2 react with excess KBr to actually produce 415 grams of Br2
Answers
The percentage of yield is 2.2%. and the weight of Br2 is 228 grams if 203 grams of CL2 react with excess KBr to actually produce 415 grams of Br2.
Weight of CL2 = 203 grams
Weight of Br2 = 415 grams
To calculate the yield percentage, we need to compare the actual yield to the theoretical yield.
We need to balance the chemical equation for the reaction between chlorine gas and potassium bromide.
Cl2 + 2 KBr = 2 KCl + Br2
We can notice that 1 mole of Cl2 reacts with 2 moles of KBr to produce 1 mole of Br2.
moles of Cl2 = 203 g / 70.90 g/mol
moles of Cl2 = 2.863 mol
moles of Br2 = (1 mol Br2 / 2 mol Cl2) × 2.863 mol Cl2
moles of Br2 = 1.432 mol
mass of Br2 = 1.432 mol × 159.81 g/mol
mass of Br2 = 228.9 g
Percentage of yield = 2.2%
Therefore, we can conclude that the weight of Br2 is 228 grams and the percentage of yield is 2.2%.
To learn more about Bromine gas
https://brainly.com/question/23784775
#SPJ4
fill in the blank
1 electronic configuration of 1531p is
2.what would be the atomic number of an atom with 6 valence element
Answers
Answer:
I didn't get you
Explanation:
Please explain