Answers
Using CH3NH2 and CH3NH3Cl, one may simulate the blood's buffering properties. A weak acid and its conjugate base, or a weak base and its conjugate acid, make up a buffer system.
Which of the following best describes the blood's buffer system?Carbonic acid and sodium bicarbonate. Hint: Human blood has a buffer of bicarbonate anion (HCO3) and carbonic acid (H2CO3) to keep the blood's pH between 7.35 and 7.45. Blood pH values higher or lower than 7.8 or 6.8 can be fatal.
Is blood an illustration of a fundamental buffer system?Bicarbonate anion and hydronium are in equilibrium with carbonic acid in this buffer. A weak acid and its conjugate base, or a weak base and its conjugate acid, make up a buffer.
To know more about acid visit:-
brainly.com/question/28175742
#SPJ1
Answer:
CH3NH2 and CH3NH3Cl
Explanation:
Methylamine (CH3NH2) is an organic base. In order to produce a basic buffer solution similar to blood, we can combine this base with a soluble salt of its conjugate acid, such as CH3NH3Cl. The solution of KOH and H2O would not be a good buffer because KOH is a strong base. The solution of HF and NaF is a buffer, but the pKa of HF is about 3.2, which is far from the pH of blood, 7.4.
Related Questions
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Choose all the answers that apply.
Which of the following energy forms are involved in a nuclear power plant?
heat
sound
mechanical
electrical
nuclear
Answers
Answer:
heat, electrical, mechanical, nuclear,sound
Explanation:
The power plant is mechanicalised so it produce heat which is in form of electrical and then we start hearing sound
The stream table shows the time needed for water to soak into the playfield soil.
Answers
Answer:
TRUE
Explanation:
plz brian list
Answer:
True
Explanation:
Plsss help im so confusing

Answers
Answer:
Refer to the picture. I managed to solve a few.

The drawing below shows a mixture of molecules:
carbon
nitrogen
oxygen
hydrogen
sulfur
chlorine
Suppose the following chemical reaction can take place in this mixture:
CS₂(g)+30₂(g) → CO₂(g) +2 SO₂(g)
Of which reactant are there the most initial moles? Enter its chemical formula:
Of which reactant are there the least initial moles? Enter its chemical formula:
Which reactant is the limiting reactant? Enter its chemical formula:

Answers
The most reactants in the system are the oxygen molecules while the least reactants in the system is carbon sulfide. As such carbon sulfide is the limiting reactant.
What is the reaction?We know that when we talk about a chemical reaction, what we are referring to is the interaction that is able to occur between the reactants and the products that are found in the system. We know that when we write down a chemical reaction, the species that are combined are the species that we can find on the left hand side and they are called the reactants. The species that we obtain in the reaction are the species that we can find on the right hand side and they are called the products of the reactions.
If we look at the reaction as it has been shown, we can see that there are ten oxygen molecules and three carbon sulfide molecules that were initially present in the system as we can see here. The entire reaction equation is given as; CS₂(g)+30₂(g) → CO₂(g) +2 SO₂(g).
Learn more about reaction equation:https://brainly.com/question/3588292
#SPJ1
Hypothesis II: Write the equation with Iron (III) Chloride and balance it: Iron + Copper (II) chloride --> Iron (III) chloride + Copper
Answers
Answer:
Fe + CuCl2 = FeCl2 + Cu
Explanation:
This is already balanced.
A solution has a pH of 10.
What is the concentration of hydrogen ions?
Answers
AlCl3 + Na2SO4
Na +
Al2(SOA)
+ NaCl
what type of reaction
Answers
What is the molarity of a solution made by adding 1.565 moles of
PbNO3 to 0.500 L?
300. M
31.3 M
3.13 M
1.56 M
Answers
Answer:
3.13M
Explanation:
Using the Molaitry equation ( M = n/v ) just plug numbers in and solve.
How many moles of potassium nitrate are produced at the same time 4.4 moles of aluminum phosphate are produced? Can you show steps for answer
Answers
Moles of potassium nitrate are produced at the same time 4.4 moles of aluminum phosphate are produced is 28.63 mole
Potassium nitrate is an inorganic salt with a chemical formula of KNO₃ it is a natural source of nitrate and has been used as a constituent for several different purposes including food preservatives, fertilizers, tree stump removal, rocket propellants, and fireworks
Here given data is
4.4 moles of aluminum phosphate
We have to find moles of potassium nitrate =?
Number of mole = mass of substances/mass of one mole
Number of mole = 126/ 4.4 moles
Number of mole = 28.63mole
So, 28.63 mole potassium nitrate are produced at the same time 4.4 moles of aluminum phosphate are produced
Know more about mole
https://brainly.com/question/7747438
#SPJ1
A template of a Venn diagram representing common and differentiating characteristics of covalent and ionic bonds is shown. Which of the following characteristics can be written only in space C?
Answers
Covalent and ionic bonds refer to atoms joined by their electrons. In covalent bonds, electrons are shared by the involved non-metal atoms. Option 2 is correct. Occurs due to the sharing of electrons between two non-metal atoms.
What are covalent and ionic bonds?
Both of them, covalent and ionic bonds, are chemical bonds that can form between atoms.
Ionic bonds occur between atoms with different electronegativity. When they bind, they transfer electrons from one atom to the other creating ions with opposite charges that attract each other.
Ionic compounds are formed by anions and cations.
• Cations are positive ions derivated from metals.
• Anions are negative ions derivated from non-metals.
The metal atoms share its electrons with the non-metal ones, creating stable configurations. Ionic bonds do not create molecules.
Covalent bonds are formed between atoms share electrons to be more stable. Atoms involved share electrons equally, creating a strong bond between them.
Covalent bonds are usually formed between non-metal atoms.
Option 2 is correct. Occurs due to the sharing of electrons between two non-metal atoms
You can learn more about covalent and ionic bonds at
https://brainly.com/question/19739192
#SPJ1
Complete question
A template of a Venn diagram representing common and differentiating characteristics of covalent and ionic bonds is shown.
Which of the following characteristics can be written only in space C?
On the diagram,
The non-overlapping space on the left is marked A, and belongs to the IONIC BOND side of the diagram.The overlapping space is marked B The non-overlapping space on the right is marked C, and belongs to the COVALENT BOND side of the diagram.Options,
Formed between positively and negatively charged ionsOccurs due to the sharing of electrons between two non-metal atomsOccurs in substances that are mostly solids at normal temperature and pressureFormed between an atom with very high electronegativity and an atom with very low electronegativityWhich element has 38 protons and 50 neutrons? __________________
What is its mass number? __________________
What group is this element in? __________________
What is the family name for this group? __________________
Answers
There are various kind of elements that are present in periodic table. Some elements are harmful, some are radioactive, some are noble gases. Therefore, Strontium belongs to group 2, alkaline earth metal family of the periodic table.
What is periodic table?Periodic table is a table in which we find elements with properties like metals, non metals, metalloids and radioactive element arranges in increasing atomic number.
Periodic table help a scientist to know what are the different types of elements are present in periodic table so that they can discover the new elements that are not being discovered yet.
Strontium is the element having 38 protons and 50 neutrons.
mass number= proton number + neutron number
=38+ 50
=88
Group number of this element is group 2. Strontium belongs to akaline earth metal family.
Therefore, Strontium belongs to group 2, alkaline earth metal family of the periodic table.
Learn more about periodic table, here:
https://brainly.com/question/11155928
#SPJ1
Which sample represents a solution?

Answers
Tyndall effect is the scattering of light by colloidal particles. Colloids are mixtures in which one substance is dispersed evenly throughout another substance.
Solutions are a type of mixture that appear homogeneous and do not exhibit the Tyndall effect. On the other hand, suspensions and colloids do not appear homogeneous and do exhibit the Tyndall effect.
Based on the information given, Sample 1 shows yes for both filtering and settling, which means it is a heterogeneous mixture, and therefore not a solution.
Sample 2 does not show the Tyndall effect and does not settle, which means it is a homogeneous mixture, and therefore a solution. Sample 3 shows the Tyndall effect but does not settle, which means it is a heterogeneous mixture, and therefore not a solution.
Therefore, the sample that represents a solution and does not exhibit the Tyndall effect is Sample 2, and the answer is A. Sample 2.
To know more about Tyndall effect, visit :
https://brainly.com/question/23487849
#SPJ1
PLS HELP!!! 15 POINTS
Which of the elements has the smallest atomic radius? Li, P, O, S
Answers
Answer:
LI
Explanation:
Given that the vapor pressure of water is 17.54 Torr at 20 °C, calculate the vapor-pressure lowering, AP, of an aqueous solution
that is 1.80 m in sucrose (C₁2H₂₂O₁1).And
calculate the vapor-pressure lowering, AP, of an aqueous solution that is 1.80 m in aluminum chloride. Assume 100%
dissociation for electrolytes.
Answers
The vapor-pressure lowering, AP, of an aqueous solution is 2.44Torr.
Mole = \(\frac{weight}{molecular weight}\)
In a 1.80m (molal) solution, 1 kilogram of the solvent contains 1.80 moles of the solute (which is water in this case).
Water's molar mass is equal to 2(atomic mass of H) + [2(1)+16] (atomic mass of O).
=18g/mol
The mole of water in 1 kilogram (equivalent to 1000 grams)::
\(n_{H_{2} O}\)= \(\frac{1000}{18g/mol}\) = 55.56mol
The solute's mole fraction in the 1.80 m solution is as follows::
\(x_{solute}\) = \(\frac{n_{solute} }{n_{H_{2}O }}\)
⇒\(x_{solute} =\) \(\frac{1.80}{55.56}\) = 0.032
a. Sucrose is a non-electrolyte, i.e., it does not undergo dissociation in the aqueous solution.
As a result, the Van't Hoff factor for sucrose is = i = 1
The lowering in vapor pressure of the solution, which is 1.80m in sucrose:
=\(ix_{solute}p^{0}\)
=(1)(0.032)(17.54)Torr
=(0.56)Torr
b. Aluminum chloride dissociate in water as follows:
\(AlCl_{3}\) →\(Al^{3+}+ 3Cl^{-}\)
As a result, an aqueous solution containing one aluminum chloride particle yields four more particles.
Thus, i=4
The lowering in vapor pressure is thus:
=\(ix_{solute}p^{0}\)
=(4)(0.032)(17.54)Torr
=(2.24)Torr
To know more about lowering in vapour pressure visit:
https://brainly.com/question/8964554
#SPJ1
4. One molecule of propanol contains a total of
flonsoona
(1) one -OH group
(2) two -CH3 groups
(3) three -OH groups
(4) three -CH3 groups
Answers
One molecule of propanol contains only one -OH group and not three -OH groups or three -CH3 groups.
The -OH group is attached to the central carbon atom and makes propanol a useful solvent and intermediate in organic chemistry.Propanol is a colorless liquid that belongs to the family of alcohols. It has the formula C3H8O, and it contains three carbon atoms, eight hydrogen atoms, and one hydroxyl group (-OH) attached to one of the carbons. One molecule of propanol contains only one -OH group, which is attached to the central carbon atom.
Thus, option (2) is the correct answer, and the other options are incorrect.The -OH group in propanol is responsible for its unique chemical and physical properties. It makes propanol soluble in water and other polar solvents and gives it a high boiling point of around 97°C. The hydroxyl group can also participate in chemical reactions, such as esterification, dehydration, oxidation, and reduction. For example, propanol can be oxidized to form propanal and then propanoic acid, which is a useful synthetic intermediate for many organic compounds.Apart from the -OH group, propanol also contains two other functional groups called methyl groups (-CH3). These are attached to the two carbon atoms adjacent to the central carbon. However, the question only asks about the number of -OH groups in propanol, so the methyl groups are irrelevant.
for such more questions on propanol
https://brainly.com/question/30086385
#SPJ8
a cube of iron pyrite is 0.31 cm on each side and has a mass of 0.040g. what is the density of the sample?
Answers
The density of the iron pyrite cube is 1.343 g/cm³.
Given,
Side of iron pyrite cube = 0.31 cm
Mass of iron pyrite = 0.040 g
The volume of iron pyrite cube = s³ cm³
Or, volume = 0.029791 cm³
We have to find the density of the sample.
Density is defined as the mass per unit volume. Or, it is the ratio of mass to the volume of the substance.
Using the formula for density, we get,
Density = mass/volume
Or, density = 0.40/0.029791
Or, density = 1.343 g/cm³
Hence, the density of the iron pyrite cube is 1.343 g/cm³.
To learn more about density, visit: https://brainly.com/question/15164682
#SPJ9
1. If 4.0 L of gas in the lungs have a pressure of 1.0 atm and are kept at body temperature (37 degrees Celsius), how many
moles are present in the lungs? Show your work.
Answers
There are approximately 0.16 moles of gas present in the lungs.
Steps
To solve this problem, we can use the ideal gas law, which relates the pressure (P), volume (V), number of moles (n), and temperature (T) of a gas:
PV = nRT
where R is the gas constant.
First, we need to convert the temperature to Kelvin:
T = 37°C + 273.15 = 310.15 K
Next, we can plug in the given values:
P = 1.0 atm
V = 4.0 L
T = 310.15 K
We also need to find the value of R, which depends on the units we are using for pressure, volume, and temperature. In this case, we are using atmospheres, liters, and Kelvin, so we can use the value:
R = 0.08206 L·atm/K·mol
Now we can rearrange the ideal gas law to solve for the number of moles:
n = PV/RT
n = (1.0 atm)(4.0 L)/(0.08206 L·atm/K·mol)(310.15 K)
n = 0.1638 mol
Therefore, there are approximately 0.16 moles of gas present in the lungs.
learn more about pressure here
https://brainly.com/question/28012687
#SPJ1
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
In a science demonstration, a teacher mixed zinc (Zn) with hydrogen chloride (HCl) in a flask and quickly attached a balloon over the mouth of the flask. Bubbles formed in the solution and the balloon inflated.
What most likely occurred during this demonstration?
a.The Zn and HCl both retained their identity.
b.Either Zn or HCl, but not both, retained its identity.
c.Evaporation of one of the substances occurred.
d.One or more new substances formed.
Answers
Answer:
a. The Zn and HCl both retained their identity.
Is anyone good at chemistry if so Is it possible could someone help me please
NO LINKS I REPEAT NO LINKS

Answers
344.0 / 68.8 = 5 (no.of half life that have elapsed)
Mass remains = (1/2)^n × (original mass)
= (1/2)^5 × 200
= (0.5) ^5 × 200
= 0.03125 × 200
= 6.25 grams
Help pls! Which of the following shows a balanced nuclear reaction?
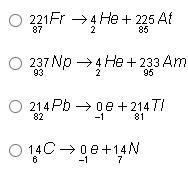
Answers
Answer:
Option 4: ¹⁴₆C —> ⁰₋₁e + ¹⁴₇N
Explanation:
To know which option is correct, do the following:
Option 1:
²²¹₈₇Fr —> ⁴₂He + ²²⁵₈₅At
87 = 2 + 85
87 = 87
221 = 4 + 225
221 ≠ 229
Thus the equation is not balanced.
Option 2:
²³⁷₉₃Np —> ⁴₂He + ²³³₉₅Am
93 = 2 + 95
93 ≠ 97
237 = 4 + 233
237 = 237
Thus, the equation is not balanced.
Option 3:
²¹⁴₈₂Pb —> ⁰₋₁e + ²¹⁴₈₁Tl
82 = –1 + 81
82 ≠ 80
214 = 0 + 214
214 = 214
Thus, the equation is not balanced.
Option 4:
¹⁴₆C —> ⁰₋₁e + ¹⁴₇N
6 = –1 + 7
6 = 6
14 = 0 + 14
14 = 14
Thus, the equation is balanced.
From the illustrations above, only option 4 is correct.
1. Why do some combinations of ionic compounds form a precipitate while others do not?
2. Solutions of lead(II) nitrate and potassium iodide were combined in a test tube. The results of this reaction are shown below.
a. Write a formula equation for the reaction.
b. Which of the possible products is the precipitate, and how do you know?
c. Write a complete ionic equation for the reaction and identify the spectator ions.
d. Write a net ionic equation for the reaction between lead(II) nitrate and potassium iodide.
Answers
Answer:
1. Some combination of ions form a solid precipitate because it is not favorable for the ions to become solvated (dissolved). Large and lowly charged ions tend to form precipitates, especially metals such as lead, barium, and silver.
2.
a. Pb(NO3)2 + 2KI -> 2KNO3 + PbI2
b. PbI2 is a precipitate because no other combinations of cations and anions will make an insoluble compound. KI, KNO3, and Pb(NO3)2 are all soluble.
c.
\(Pb^{2+}(aq) + 2NO_3^{-}(aq) + 2K^+(aq) + 2I^-{aq} = > PbI_2(s) + 2NO_3^{-}(aq) + 2K^+{(aq)}\\\\\)
is the ionic equation. Spectator ions are NO3- and K+
d.
\(Pb^{2+}(aq)+ 2I^-{aq} = > PbI_2(s) \\\) is the net ionic equation
ask questions in comments if you have any
Rank the following compounds in order of decreasing vapor pressure.
CH3CH2CH2CH2CH3
CH4
CH3CH-CH3CH2CH3
CH3CH2CH2OH
Answers
Decreasing vapour pressure for the following compounds is as follow:
CH4
CH3CH-CH3CH2CH3
CH3CH2CH2CH2CH3
CH3CH2CH2OH
Vapour Pressure of the compound:
The pressure characteristic of a pure compound's vapour at any given temperature when it is in equilibrium with its liquid or solid state is known as the vapour pressure. Compound molecules that bond well with one another will have a low vapor pressure (less inclination to escape to the vapor phase), whereas compounds that connect poorly with one another would have a high vapor pressure. Vapor pressure is a measure of a compound's capacity to bond with itself.
To learn more about the Vapor Pressure:
https://brainly.com/question/4463307
#SPJ1
Which of the following has the correct order of increasing relative strengths? A. Hydrogen bonding < covalent bonds < dipole force < dispersion forceB. Dispersion force < dipole force < hydrogen bonding < covalent bondingC. covalent bond < hydrogen bond < dipole < dispersionD. Covalent bonds < hydrogen bond < dispersion < dipole force
Answers
Explanation:
First, we need to know that chemical bonds such as covalent and ionic bonds will always have greater strengths in relation to intermolecular interactions.
Hydrogen bond will be the intermolecular interaction with the greates streght. Then dipole force and then dipole.
Answer: B. Dispersion force < dipole force < hydrogen bonding < covalent bonding
How many atoms of Oxygen are represented below?
2C9H8O4
Answers
Explanation:
nnnnnnnmnnmmnnnn
eight
You have two samples of substances that look the same - both white crystalline structures. You know that one of the substances is sucrose, C12H22O11 , and the other is table salt, NaCl, but you’re not sure how to tell them apart!
a.) How can you use a conductivity tester to differentiate between the two substances? b.) How can you use a hot plate to differentiate between the two substances?
Answers
The sodium chloride solution would melt at a high temperature and conduct electricity but the sucrose would not do any of these.
What is conductivity test?The conductivity test is the test that can be use to determine which of the substances can be able to conduct electricity. The substance that can be able to conduct electricity must be ionic in nature and would also have a high melting point.
a) If we dissolve the two substances in water and then connect an external cell to it, we would notice that the sodium chloride solution would conduct electricity but the sucrose solution would not.
b) On the other hand, the sodium chloride solution would be found to melt at a much higher temperature than the sucrose.
Learn more about conductivity:https://brainly.com/question/21496559
#SPJ1
1. What type of organic compounds are the reactants in an esterification reaction?
Answers
Esterification is the process that turned salicylic acid and acetic acid into acetylsalicylic acid.
Thus, The process of creating esters from carboxylic acids is known as esterification. When a carbon is linked to two oxygen atoms, an ester results from one of the oxygens being double-bonded to the carbon while the other oxygen is linked to another carbon.
The letter "R" stands for a carbon chain in the following generic formula for an ester.
Esterification occurs when a carboxylic acid reacts with an alcohol. This reaction can only occur in the presence of an acid catalyst and heat. It takes a lot of energy to remove the -OH from the carboxylic acid, so a catalyst and heat are needed to produce the necessary energy.
Thus, Esterification is the process that turned salicylic acid and acetic acid into acetylsalicylic acid.
Learn more about Esterification, refer to the link:
https://brainly.com/question/31118260
#SPJ1
How much heat is gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C? The specific heat of nickel is 0.443 J/g · °C.
Answers
Explanation:
To calculate the heat gained by nickel, we can use the formula:
q = m * c * ΔT
where q is the heat gained, m is the mass of the nickel, c is the specific heat of nickel, and ΔT is the change in temperature.
Given:
- Mass of nickel, m = 31.4 g
- Specific heat of nickel, c = 0.443 J/g · °C
- Change in temperature, ΔT = 64.2 °C - 27.2 °C = 37.0 °C
Substituting the values into the formula, we get:
q = (31.4 g) * (0.443 J/g · °C) * (37.0 °C)
Simplifying the calculation, we get:
q = 584 J
Therefore, the heat gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C is 584 J.
2,3,7,9-tetramethyl-5-(1-metilbutil)decano
Answers
The IUPAC name for 2,3,7,9-tetramethyl-5-(1-methylbutyl)decanoic acid is 2,3,7,9-tetramethyl-5-(1-methylbutyl)decanoic acid. It is a carboxylic acid with a molecular formula of C20H40O2 and a molecular mass of 312.54 g/mol. The compound has a melting point range of 70-75°C.
2,3,7,9-tetramethyl-5-(1-methylbutyl)decanoic acid contains a carboxyl group (-COOH), which is the functional group responsible for its acidic properties and solubility in water. The presence of the carboxyl group makes the compound more polar than similar molecules, enhancing its water solubility. Carboxylic acids are also known for their reactivity with bases and ability to undergo esterification reactions.
In summary, 2,3,7,9-tetramethyl-5-(1-methylbutyl)decanoic acid is a carboxylic acid with a unique molecular structure. Its IUPAC name describes the positions and nature of the substituent groups. The presence of the carboxyl group contributes to its polarity, water solubility, acidity, and reactivity with bases and esterification reactions. The compound's characteristics are significant in understanding its properties and potential applications in various chemical reactions and processes.
for such more questions on compound
https://brainly.com/question/29108029
#SPJ8