Answers
Answer:
Ca = 40.1 g
Cl = 35.5
So CaCl240.1 + 35.5×2
40.1 + 71
111.1 g
Related Questions
Which of the following is not a characteristic of a sonnet?
14 lines
Blank verse
Iambic pentameter
Rhyme scheme ABAB CDCD EFEF GG
Answers
Blank verse is not a characteristic of a sonnet.
What is not a characteristics of a sonnet?Blank verse is not a characteristic of a sonnet. Sonnets are typically written in iambic pentameter and have 14 lines, and the rhyme scheme varies depending on the type of sonnet. For example, the Shakespearean sonnet typically has the rhyme scheme ABAB CDCD EFEF GG, while the Petrarchan sonnet has the rhyme scheme ABBAABBA CDCDCD or ABBAABBA CDECDE. Blank verse, on the other hand, is unrhymed iambic pentameter and is typically used in longer poems or plays.
Read more on sonnets here:https://brainly.com/question/16369162
#SPJ1
balance equation of calcium hydroxide+carbon dioxide= calcium carbonate+water for grade 8 in step wise
Answers
Answer:
Ca(OH)2 +CO2========CaCO3 +H2O
Explanation:
since the number of elements in the right is equal to that of the elements in the left side shows it is balanced
5. What is the systemic name for the following structure?
Answers
Answer:
You are not showing the question, but I believe the answer is cis-3,4-dimethyl-3-hexene.
Explanation:
since the substituents are on same side, it call cis. Followed by the name.
What state of matter is Polaris, the north star?
A. Plasma
B. Liquid
C. Light
D. Gas
Answers
The state of matter of Polaris, the north star is gas.
What are stars made of?Stars are made up of a mixture of hot gases.
The mixture consists of helium and hydrogen. Hydrogen burns into helium to give starts a shining appearance when observed from a far distance.
Thus, the state of matter of all stars, including the north star, is gas.
More on stars can be found here: https://brainly.com/question/21521087
#SPJ1
Which of these molecules is polar?
A. O₂
B. N2
C. BH3
D. NO
Answers
Explanation:
Then there is no net charge separation. a BH3 b CHCl3 c C2H2 d NH3. Optionally the ... Oxygen O2 c. ... BH3 2. Com Bh3 Polar Or Nonpolar In the case of non polar molecules dispersion forces or London forces are present between them.
The polar molecules is NO.
What is polar molecule?A polar molecule is one that has one end that is slightly positive and the other end that is slightly negative. Ionic or polar covalent bonds can form in polar molecule. A dipole is a molecule that has two poles. The dipole moment is the outcome of measuring the amount of polarity in a molecule.
NO molecule, In the periodic table, nitrogen is to the right of oxygen. Nitrogen has a lower electronegative potential than oxygen. All N-O bonds are polar, with the oxygen atom having a higher electron density. The uneven distribution of electrons in the NO molecule is quite minor. When a molecule is non-polar, the electrons are shared evenly, resulting in a non-polar bond, or the polar bonds are symmetric. So, oxygen, nitrogen and boron trihydride molecules are non-polar molecule.
Hence the correct option is D.
To know more about polar molecule, here,
https://brainly.com/question/11405437
#SPJ2
What's a house hold item that has nuclear energy
Answers
Answer:
Electricity, Weapons, Medicine · Food Treatments, ect.
Guysss how to explain nuclear chemistry? And define nuclear chemistry ?
Answers
Answer:
How do amoeba respire.
Define Diffusion.
vitamin A in chemistry
Answers
Answer:
\(\sf\fbox\red{Answer:-}\)
Vitamin A is a fat-soluble vitamin and an essential nutrient for humans. It is a group of organic compounds that includes retinol, retinal (also known as retinaldehyde), retinoic acid, and several provitamin A carotenoids (most notably beta-carotene (β-carotene).
\(\small\fbox{\blue{\underline{mαrk \; mє \; вrαínlíєѕt \; plєαѕє ♥}}}\)
What mass (grams) of antimony(III) chloride would be produced by reacting with 112 liters of chlorine measured at STP?
Answers
Answer:
radius = 16 in ; height = 27 in
Explain how physical and chemical properties influence the formation of ocean current and the distribution of marine life.
False answers will be reported; a good answer will be given brainliest.
Answers
The physical and chemical properties of seawater vary according to latitude, depth, nearness to land, and input of fresh water. The physical and chemical properties of seawater have a great effect on organisms, varying especially with the size of the creature. Marine organisms have evolved a wide variety of unique physiological and morphological features that allow them to live in the sea.
Hopefully Help:)
#CarryOnLearningCassini has a mass of 2523 kg, and Saturn
has a mass of 5.68 x 1026 kg. Saturn's radius
is 54,364 km. If Cassini feels a gravitational
force of 2.980 x 104 N, how high above
Saturn's surface is it?
Rearrange F gravity Gm,m₂/r2
to solve this problem
In 10 words or fewer, how high above Saturn's surface is the Cassini
satellite?
Answers
The F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and height is 108,728 km.
What is gravity?Gravity is the amount of force that is produced by the earth to attract the object toward the surface and it doubles if the mass is double.
The height of Saturn is the duble of the radius of the given radius of 54,364 km of the planet Saturn which is 108,728 km.
Therefore, F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and hight is 108,728 km.
Learn more about gravity, here:
https://brainly.com/question/4783082
#SPJ1
The reactant concentration in a zero-order reaction was 8.00×10−2 M
after 140 s and 4.00×10−2 M after 400 s
. What is the rate constant for this reaction?
Answers
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1, depending on which rate was used to calculate it.
Determining the rate constantThe rate of the reaction is given by the equation:
Rate = -k[A]
where k is the rate constant and [A] is the concentration of the reactant.
Rate at t=140 s:
Rate = (8.00×10−2 M - 0 M) / (140 s - 0 s)
= 5.71×10−4 M/s
Rate at t=400 s:
Rate = (4.00×10−2 M - 0 M) / (400 s - 0 s)
= 1.00×10−4 M/s
Since this is a zero-order reaction, the rate of the reaction is constant, and we can use either rate to calculate the rate constant:
k = Rate / [A]
Using the rate at t=140 s:
k = 5.71×10−4 M/s / 8.00×10−2 M = 7.14×10−3 s−1
Using the rate at t=400 s:
k = 1.00×10−4 M/s / 4.00×10−2 M
= 2.50×10−3 s−1
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1.
Learn more on zero-order reaction https://brainly.com/question/21663229
#SPJ1
Balance the equations by inserting coefficients as needed.
equation 1:
PCl_{3} + Cl_{2} -> PCl_{5}
PCl3+Cl2⟶PCl5
equation 2:
Mg_{3}N_{2} + HCl -> MgCl_{2} + NH_{3}
Mg3N2+HCl⟶MgCl2+NH3
Answers
Answer:
first one is already balanced
2. MgN2+6HCl->3MgCl2+2NH3
Explanation:
To balance the equations, we need to ensure that the same number of each type of atom is present on both sides of the equation. Here's how we can balance the given equations:
Equation 1: PCl₃ + Cl₂ -> PCl₅
In this equation, there are 1 phosphorus (P) atom, 3 chlorine (Cl) atoms, and 5 chlorine (Cl) atoms. To balance the equation, we can put a coefficient of 2 in front of PCl₃ to have 2 phosphorus atoms, and a coefficient of 5 in front of Cl₂ to have 10 chlorine atoms:
2PCl₃ + 5Cl₂ -> PCl₅
Now the equation is balanced with 2 phosphorus atoms and 10 chlorine atoms on both sides.
Equation 2: Mg₃N₂ + HCl -> MgCl₂ + NH₃
In this equation, there are 3 magnesium (Mg) atoms, 2 nitrogen (N) atoms, 2 hydrogen (H) atoms, and 1 chlorine (Cl) atom.
To balance the equation, we can put a coefficient of 3 in front of HCl to have 3 hydrogen atoms, and a coefficient of 2 in front of NH3 to have 2 nitrogen atoms:
Mg₃N₂ + 3HCl -> MgCl₂ + 2NH₃
Now the equation is balanced with 3 magnesium atoms, 2 nitrogen atoms, 6 hydrogen atoms, and 2 chlorine atoms on both sides.
Know more about coefficient:
https://brainly.com/question/12318261
#SPJ2
In Part A, we saw that the theoretical yield of aluminum oxide is 1.60 mol . Calculate the percent yield if the actual yield of aluminum oxide is 1.22 mol .
Answers
Taking into account definition of percent yield, the percent yield for the reaction is 76.25%.
Percent yieldThe percent yield is the ratio of the actual return to the theoretical return expressed as a percentage.
The percent yield is calculated as the experimental yield divided by the theoretical yield multiplied by 100%:
\(percent yield=\frac{actual yield}{theorical yield}x100\)
where the theoretical yield is the amount of product acquired through the complete conversion of all reagents in the final product, that is, it is the maximum amount of product that could be formed from the given amounts of reagents.
Percent yield in this caseIn this case, you know:
actual yield= 1.22 moltheorical yield= 1.60 molReplacing in the definition of percent yields:
\(percent yield=\frac{1.22 mol}{1.60 mol}x100\)
Solving:
percent yield= 76.25%
Finally, the percent yield for the reaction is 76.25%.
Learn more about percent yield:
brainly.com/question/14408642
#SPJ1
Two solutions are separated by a semipermeable membrane. On one side of the semipermeable membrane is a 1.5 M NaCl solution. On the other side of the membrane there is a 0.5 M KBr solution. Which statement describes the direction water will flow?
Answers
If two solutions are separated by a semipermeable membrane with one side 1.5 M NaCl solution and other side 0.5 M KBr solution. Here water will flow from the KBr solution to the NaCl solution because of the water concentration difference.
When two solutions are separated with a semipermeable membrane, the water will flow from lower solute concentrated side to higher solute concentrated side.
Lower solute concentration means the water content will be high and that states higher water concentration.
Higher solute concentration means the water content will be low and that states low water concentration.
The water will flow from higher concentration to lower till the equilibrium where both sides the water and solute concentration is same.
In this case, the lower solute concentration is the 0.5 M KBr solution and the higher solute concentration is the 1.5 M NaCl solution.
Therefore, water will tend to flow from the KBr solution to the NaCl solution.
Learn more about concentration:
https://brainly.com/question/12417124
#SPJ4
Hey can anyone pls solve these problems!!! pls

Answers
2. A restaurant offers a $19.99 three course meal special that lets you choose an appetizer,
an entree, and a dessert. There are 8 appetizers, 12 entrees, and 6 desserts from which
to choose. How many different meals are available?
Answers
There are 576 different ways to choose a meal.
Promotional offers. A promotional offer is a specific proposition to clients that specifies a reward and patron behavioral standards for earning a reward. the recognition and trouble of the praise are captured thru a retail transaction, purchaser order, rebate claim, rebate redemption, or different patron interplay. a discount is a difference between the unique charge and the lower charge it's far being bought at. a proposal is a deal wherein a product is normally bought at a reduction.
Calculation:-
There are 8 appetizers, 12 entrees, and 6 desserts.
Number of choices = 8 × 12 × 6
= 576
Learn more about different meals here:-https://brainly.com/question/8880115
#SPJ1
List the 2 pKa's for H2SO4
Answers
What is the chemical reaction for calcium
Answers
Answer:
I think the answer is calcium reacts with oxygen, forming a thin layer of CaO
Explanation:
I hope this helps
what carbonyl compound and grignard reagent could be used to prepare 2-butanol?
Answers
Acetone and methylmagnesium chloride could be used to prepare 2-butanol by reacting the carbonyl compound, acetone, with the Grignard reagent, methylmagnesium chloride, in the presence of aqueous acid to form 2-butanol as the primary alcohol product.
The Grignard reagent, methylmagnesium chloride, is a strong nucleophile that can attack the electrophilic carbon atom of the carbonyl group in acetone. This reaction takes place in the presence of aqueous acid, which acts as a proton source and helps to facilitate the formation of the alcohol product.
As the reaction proceeds, the carbonyl group is reduced to an alcohol and the Grignard reagent is consumed. The primary alcohol product, 2-butanol, is formed in high yield. Additionally, the reaction is reversible so the reaction is usually run under anhydrous conditions to suppress the reverse reaction.
To learn more about Grignard reagent visit: https://brainly.com/question/16040954
#SPJ4
Quickly please! Which is a group of tissues that work together to carry out a common function?
cell
organ
organelle
organ system
Answers
Oxidation number for S in the compound
ZnSO3
Answers
In ZnSO3, O is oxidized to a value of two. S in ZnSO3 is oxidized to +4 by zinc. ZnSO3 has a +2 Zn oxidation number.
What do you mean by "composite"?When two or more separate chemical components are mixed in a specific proportion, the resultant substance is called a compound. The collision of the components creates biochemical connections that have been complex to break.
Which are they—elements or compounds?There is just one sort of atom per element, making them pure substances. When a variety of different type of elements are combined chemically in predetermined ratios, the resultant product is called a compound. The Earth naturally has about 94 of the almost 118 elements that make up the periodic table.
To know more about Compound visit:
https://brainly.com/question/19458442
#SPJ1
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
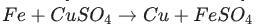
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
Calculate the mass of a sample of fruit sugar, fructose,
C6H1206, containing 2.65 X 1020 molecules.
Answers
Answer:
, containing 2.65 X 1020 molecules.
Explanation:
The mass of a sample of fruit sugar (fructose) C₆H₁₂O₆, containing 2.65 ×10²⁰ is equal to 2.88 g.
What is Avogadro's number?Avogadro’s number can be defined as the number of units in one mole of any chemical substance. Generally, these entities can be electrons, molecules, atoms, ions, or protons, depending upon the kind of chemical reaction or reactants and products.
The value of Avogadro’s constant is found approximately 6.022 × 10²³ mol⁻¹.
Given, the number of molecules of fructose = 2.65 ×10²⁰
The molar mass of the fructose = 180.16 g/mol
So 6.022 × 10²³ molecules of fructose have mass = 180.16 g
Then 2.65 × 10²⁰ fructose molecules will have mass = 2875.04 × 10⁻³ g
The mass of the 2.65 × 10²⁰ fructose molecules = 2.88 g
Therefore, the mass of 2.65 × 10²⁰ fructose molecules is equal to 2.88 g.
Learn more about Avogadro's number, here:
brainly.com/question/11907018
#SPJ2
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
Which image represents ether? Check all that apply.

Answers
Answer:
A) and D)
Explanation:
I just completed this assignment and these are the correct options!
In the given image, the compounds that represents ether are option 1 and 4, respectively.
Ether is a class of organic compounds that contain an oxygen atom bonded to two alkyl or aryl groups. The general formula for ethers is \(\rm R-O-R'\), where R and R' are alkyl or aryl groups.
Compound 1 is 1-methoxypentane is an ether with the molecular formula \(\rm C_6H_{14}O\). It consists of a pentane chain (a straight chain of five carbon atoms) with a methoxy \(\rm (-OCH_3)\) group attached to the first carbon atom. Compound 4 is methoxyethane. It is an ether with the chemical formula \(\rm C_3H_8O\). The structure of methoxyethane consists of a ethane chain (a straight chain of two carbon atoms) with a methoxy \(\rm (-OCH_3)\) group attached to the first carbon atom.Therefore, compound 1 and compound 4 represents ether. The correct answer is option 1 and 4, respectively.
Learn more about Ether here:
https://brainly.com/question/28047849
#SPJ6
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
The temperature at which a solid melts is the same as the temperature at which its liquid form solidifies (true or false)
Answers
its false not true................
Answer:
True
Explanation:
This is true because, upon cooling the particles in a liquid loses energy, stops moving and remains constant and the forms a solid.
Therefore we can say the freezing point and melting point occurs at the same temperature
The half-life of a radioactive isotope is 27 years. How long will its
mass take to fall from 2.00 g to 0.25 g?
years.
Answers
Life will come out to be 10 days. This is the required value of half life.
what is radioactive isotope?
An unstable form of a chemical element that releases radiation as it breaks down and becomes more stable. Radioisotopes may occur in nature or be made in a laboratory. In medicine, they are used in imaging tests and in treatment. Also called radionuclide. Radioactive isotopes have many useful applications. In medicine, for example, cobalt-60 is extensively employed as a radiation source to arrest the development of cancer. Other radioactive isotopes are used as tracers for diagnostic purposes as well as in research on metabolic processes.Atoms of the same element with different numbers of neutrons are called isotopes. Many elements have one or more isotopes that are radioactive. Their nuclei are unstable, so they break down, or decay, and emit radiation.To learn more about isotope refers to:
https://brainly.com/question/364529
#SPJ1