Convert 3.50 mm to m. Report your answer to the appropriate number of significant figures. 1 m= 1000 mm
Answers
Answer:
0.0035
Explanation:
3.5 millimetres = 0.0035 meters
Answer:
0.0035 m
Explanation:
since 1000 mm is equal to 1 m
then 3.50 mm will be equal to lets say x
1000 mm = 1 m
3.50 mm = x m
cross multiply
1000 × x = 3.50 × 1
divide both sides by 1000
x = 3.50/1000
x= 0.0035 m
Related Questions
how is oxygen transported in the blood?multiple choice10-20% dissolved in plasma; 80-90% as oxyhemoglobin98-99% dissolved in plasma; 1-2% as oxyhemoglobin50% dissolved in plasma; 50% as oxyhemoglobin1-2% dissolved in plasma; 98-99% as oxyhemoglobin
Answers
The correct answer is A. 10-20% of oxygen is dissolved in plasma and 80-90% is transported as oxyhemoglobin in the blood.
Oxygen is transported in the blood primarily through a combination of dissolved oxygen in plasma and oxygen bound to hemoglobin within red blood cells. Approximately 10-20% of oxygen is found dissolved in plasma, while the remaining 80-90% is bound to hemoglobin in the form of oxyhemoglobin. The concentration of oxygen in plasma is determined by the partial pressure of oxygen in the environment, while the amount bound to hemoglobin is determined by the amount of hemoglobin present in the blood. Oxygen can also be transported in the form of bicarbonate and other small molecules.
learn more about bicarbonate refer: https://brainly.com/question/29855231
#SPJ11
complete question: how is oxygen transported in the blood?multiple choice
A.10-20% dissolved in plasma; 80-90% as oxyhemoglobin
B.98-99% dissolved in plasma; 1-2% as oxyhemoglobin
C.50% dissolved in plasma; 50% as oxyhemoglobin
D. 1-2% dissolved in plasma; 98-99% as oxyhemoglobin
what feature related to the composition of a comopund can be determined solely by percent composisi
Answers
The % composition of a substance can be used to calculate empirical formulas. The molar mass of the chemical must also be known in order to determine its molecular formula.
What is empirical formula explain?
A compound's various atoms are arranged in an empirical formula in the simplest whole-number ratio.
The exact amount of various atom types that make up a compound's molecule are displayed in the molecular formula. The empirical formula for acetylene is CH. Example: C2H2 is the empirical formula for acetylene.
An illustration of an empirical formula?
Its chemical name is C6H12O6, and it is a simple sugar. Every mole of carbon and oxygen is accompanied by two moles of hydrogen. Glucose's empirical formula is CH2O.
Learn more about empirical formula
brainly.com/question/14044066
#SPJ4
Which atoms are likely to form stable molecules that have an incomplete octet on the central atom?.
Answers
Atoms that are likely to form stable molecules that have an incomplete octet on the central atom are ones that have less than eight valence electrons in their outermost shell. Atoms of this type can achieve stability by forming covalent bonds with other atoms to share electrons in a way that fills their valence shells.
There are several types of atoms that can do this, including boron, beryllium, and aluminum. These atoms typically form molecules in which they are the central atom, and they may also bond with other atoms to form ions with incomplete octets. When it comes to molecular structures, the octet rule is a basic principle. This rule states that atoms tend to form stable molecules by gaining, losing, or sharing electrons to achieve a full octet of eight valence electrons in their outermost shell.
This configuration is believed to be the most stable for most atoms, so molecules that follow this principle are more likely to be stable and chemically inert. However, there are certain atoms that do not follow the octet rule. These atoms, which have less than eight valence electrons in their outermost shell, are called incomplete octets. In order to become more stable, these atoms need to form covalent bonds with other atoms so that they can share electrons to fill their valence shells. This allows them to form molecules that have an incomplete octet on the central atom.
In conclusion, atoms that have less than eight valence electrons in their outermost shell are likely to form stable molecules that have an incomplete octet on the central atom. These atoms can achieve stability by forming covalent bonds with other atoms to share electrons, which allows them to fill their valence shells. Examples of atoms that can do this include boron, beryllium, and aluminum. While molecules with incomplete octets are not as stable as those that follow the octet rule, they are still important in chemical reactions and play an important role in the chemistry of certain elements.
To know more about valence electrons visit:
brainly.com/question/31264554
#SPJ11
When 50g of sugar is dissolved in 100g mL of water there is no increase in volume what characteristics is illustrated by this observation?
Answers
This observation illustrates the concept of a solution's solubility, which is the amount of solute that can be dissolved in a given amount of solvent at a certain temperature. In this case, the sugar is said to be highly soluble in water, as it was able to dissolve completely in the given volume.
What is solubility?Solubility is the property of a substance to dissolve in a solvent to form a homogeneous solution. It is a measure of the maximum amount of solute that can be dissolved in a given amount of solvent at a given temperature and pressure. Solubility is an important factor in many areas of science, including chemistry, pharmacy and environmental science.
This observation also shows that the sugar does not take up any additional volume when dissolved, as its molecules are small enough to disperse evenly throughout the solvent.
To know more about solubility click-
https://brainly.com/question/23946616
#SPJ4
Someone pls help me I will mark you as brain
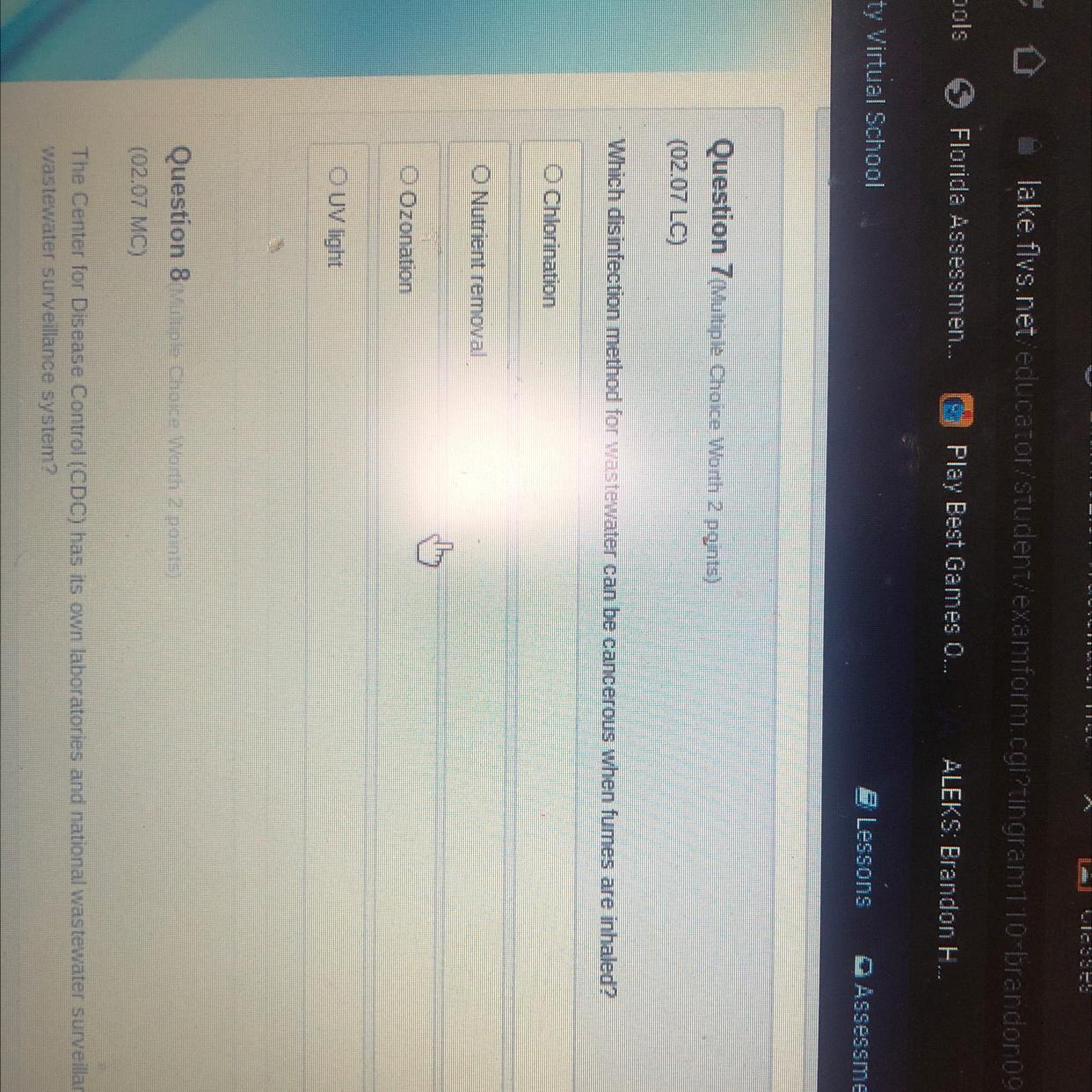
Answers
Answer:
Chlorination
Explanation:
"Exposure to high volumes of chlorine gas fumes can cause serious health problems, including death."
https://water.mecc.edu/courses/ENV211/lesson14_print.htm
:)
what is the ph of a solution that has an h concentration of: 1.75 x 10-5 mol/l 6.50 x 10-10 mol/l 1.0 x 10-4 mol/l 1.50 x 10-5 mol/l
Answers
The pH for each of the given H+ concentrations are:
1.75 x 10⁻⁵ mol/l = 4.766.50 x 10⁻¹⁰ mol/l = 9.191.0 x 10⁻⁴ mol/l = 41.50 x 10⁻⁵ mol/l = 4.82The pH of a solution can be calculated using the formula: pH = -log[H+], where [H+] is the concentration of hydrogen ions in moles per liter.
Using this formula, we can find the pH for each of the given H+ concentrations:
Learn more about pH for H+ concentrations at https://brainly.com/question/28444954
#SPJ11
PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!!PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!! PLSSS HELPPPP I WILLL GIVE YOU BRAINLIEST!!!!!!

Answers
Answer:
Full answer in explanation
Explanation:
High Tide - when tides are at their highest elevationLow Tide - when tides are at their lowest elevationSpring Tide - when tides experience the greatest range between high and low; occur at New and Full MoonsNeap Tide - when tides experience the least range between high and low; occur at 1st and 3rd Quarter MoonsGravitational Pull - the invisible force that exists between all objects that have mass. The greater the mass, the greater the effect of the forceTide - the daily change in ocean levels due to the gravitational force of the moon and the sun exerted on EarthHope this helps!
Calculate the equilibrium concentrations of n2o4 and no2 after the extra 1. 00 mol no2 is added to 1. 00 l of solution.
Answers
The equilibrium concentrations of N2O4 and NO2 after the addition of 1.00 mol of NO2 to 1.00 L of solution are 0.316 M and 1.684 M, respectively.
In the chemical equilibrium, the concentrations of the products and reactants remain constant. At equilibrium, the concentration of N2O4 is given by the formula below:N2O4(g) ⇌ 2NO2(g)Kc = [NO2]2/[N2O4]Taking the equilibrium concentration of N2O4 as x and the concentration of NO2 as (1.00 – x) .
The concentrations are expressed in mol/L as both the volume and moles of NO2 added are 1.00. The concentration of N2O4 is calculated using the formula below:N2O4(g) ⇌ 2NO2(g)Kc = [NO2]2/[N2O4]Kc = [0.5]2/ [x]Kc = 0.25 / x0.25 / x = 4/xx = 4/0.25 = 16 mol/LThus, the equilibrium concentration of N2O4 is 16 mol/L - 0.5 mol/L = 0.316 mol/L while the equilibrium concentration of NO2 is 1.00 mol/L + 0.5 mol/L = 1.684 mol/L.
To know more about equilibrium visit:
https://brainly.com/question/30694482
#SPJ11
how many equivalent statements are needed to convert 23.4 Gm into mm? 20 points!
Answers
Answer:
23.4 x 10¹²mm
Explanation:
Given problem;
Convert Gm to mm;
Gm = Gigameters
mm = millimeters
1 Gigameter = 1 x 10⁹m
1000mm = 1 m
To convert from Gm to mm;
23.4 Gm x \(\frac{1 x 10^{9} m}{1Gm}\) x \(\frac{1000mm}{1m}\) = 23.4 x 1 x 10⁹ x 10³ =
= 23.4 x 10¹²mm
Which is similar to argon? Al3+ Cl1- Cl Mg2+
Answers
Answer:
Cl⁻
Explanation:
The chlorine ion Cl⁻ is the most similar to Argon from the given choices.
Argon is an element with 18 electrons.
It is a noble gas and so does not readily combine with other atoms.
Now,
For an atom to be similar to argon, it must have the same number of electrons which is 18.
Cl⁻ is a chloride ionIt has gained one electron to its initial 17 electrons thus making the number 18 The net charge is a negative ion.At room temperature I2(s) is a molecular solid. Which of the following provides a characteristic of I2(s) with a correct explanation?O It has a high melting point because it has weak intermolecular forces.
O It is hard because it forms a threedimensional covalent network.
O It is not a good conductor of electricity because its valence electrons are localized in bonding and nonbonding pairs.
O It is very soluble in water because its molecules are polar.
Answers
Due of the weak intermolecular interactions, it has a high melting point.
A force that attracts the protons or positive parts of one molecule to the electrons or negative parts of another molecule is known as an intermolecular force. A substance's various physical and chemical properties are influenced by this force. The strength of an object's intermolecular forces determines its boiling point; the higher the intermolecular forces, the higher the boiling point.
We can compare the intermolecular forces between different substances by comparing their boiling points. This is so that these intermolecular interactions can be broken and the liquid can be transformed into vapour using the heat that the substance absorbs at its boiling point.
To know more about intermolecular forces
https://brainly.com/question/9007693
#SPJ4
C. Use the data provided in Table 2 to complete the following.
Sketch a phase diagram for O₂. The diagram should be roughly to
scale and include the Triple point and Critical point.

Answers
The triple point can be seen from the graph that has been attached here.
What is the triple point?The triple point is a special combination of temperature and pressure where the equilibrium of a substance's three phases—solid, liquid, and gas—occurs. The transition between phases happens with no discernible net change in the substance because the solid, liquid, and gas phases are in perfect equilibrium at the triple point.
A crucial reference point in thermodynamics, the triple point is frequently used to specify temperature scales and calibrate thermometers. Each substance has a specified value for temperature and pressure.
Learn more about triple point:https://brainly.com/question/29017350
#SPJ1

Which of the following is the best example of potential energy?
A bird making a nest
A car stopped at the top of a hill
A gymnast doing a cartwheel
A swimmer doing the backstroke
Answers
A car stopped at the top of a hill
Explanation :
Definition of potential energy : energy stored that depends upon the relative position of various parts of a system
What is the identity of element Y?
OOOO
C
Ов
Na
S
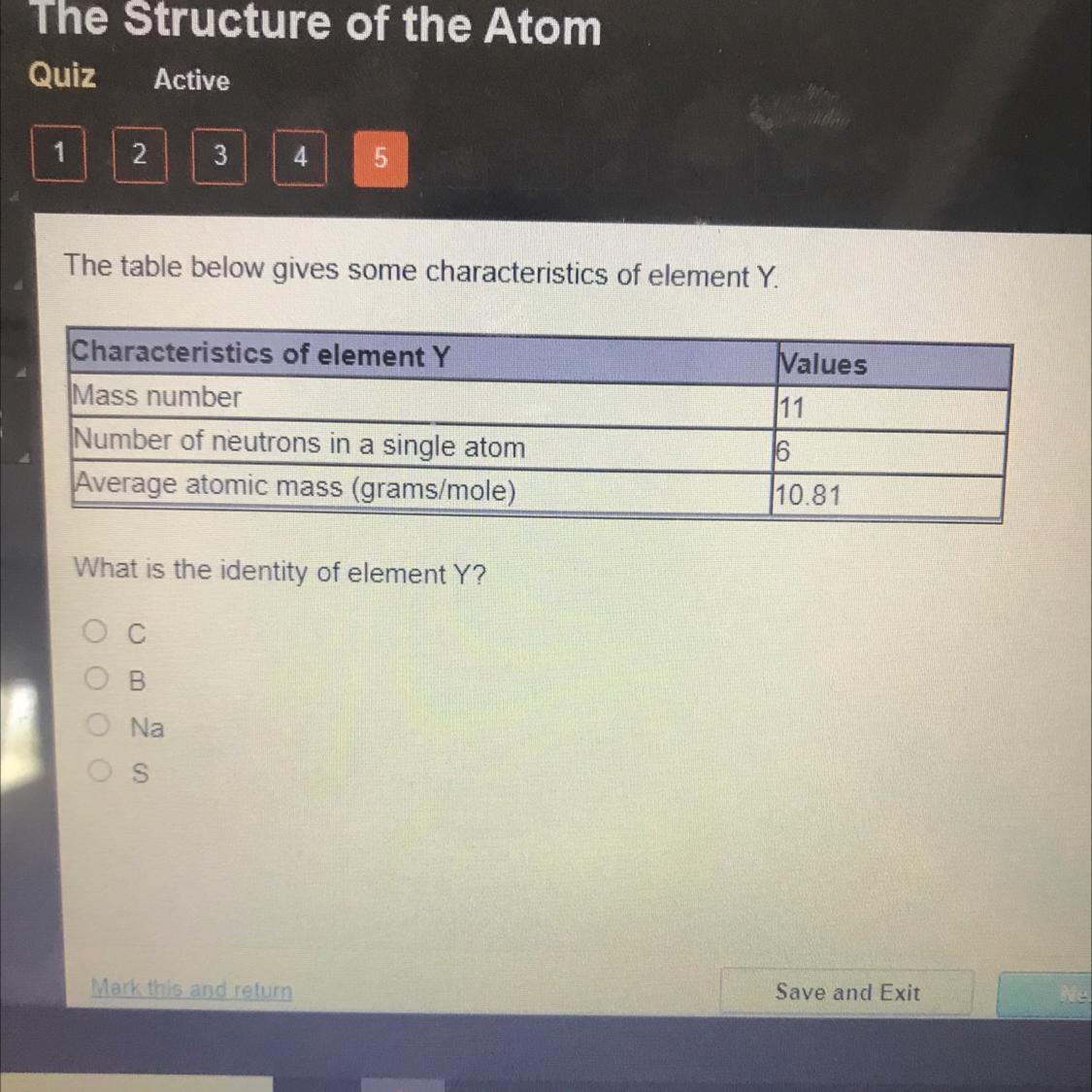
Answers
Answer: Boron
Explanation: its average atomic mass on the periodic table is 10.8
Answer: C
Explanation:
according to collision theory, what is/are the essential qualification(s) that must occur for a reaction to form products? choose one or all that apply. not all molecular collisions lead to the formation of products. in fact, only a small fraction of collisions lead to product formation. according to collision theory, what is/are the essential qualification(s) that must occur for a reaction to form products? choose one or all that apply. reacting particles must have the proper orientation. reacting particles must only be gaseous. reacting particles must have sufficient mass. reacting particles must collide. reacting particles must have sufficient energy.
Answers
According to collision theory, the essential qualification(s) that must occur for a reaction to form products are reacting particles must have the proper orientation, reacting particles must collide and reacting particles must have sufficient energy. Therefore, option A, D and E are correct.
What is collision theory ?According to collision theory, for a chemical reaction to occur, the reacting particles must collide with one another. The reaction rate is determined by the frequency of collisions. According to the theory, reacting particles frequently collide without reacting.
Collision theory, as the name implies, describes how collisions, specifically collisions between particles, result in chemical change. The formation of entirely new substances is referred to as chemical change.
Thus, option A, D and E are correct.
To learn more about the collision theory, follow the link;
https://brainly.com/question/14566831
#SPJ1
balancing equations help!!

Answers
2) 2KClO3–> 2KCl+3O2
3) 2NaCl+F2–> 2NaF+Cl2
4)Pb(OH)2+ 2HCl—> 2H2O+PbCl2
5) 2AlBr3+ 3K2SO4–> 6KBr+Al2(SO4)3
6) is already balanced
If you have any questions just let me know :)
What is not a possible source of error?
Question 4 options:
people observe things slightly differently and can make mistakes
all answers are sources of error
measuring devices have limits, might be broken, or might be calibrated incorrectly
Lap equipment is not always identical (they can vary)
outside factors can affect te experiment (temperature, humidity, light, wind, etc.)
Answers
All the choices in the question are sources of error i.e.
People observe things slightly differently and can make mistakesMeasuring devices have limits, might be broken, or might be calibrated incorrectlyOutside factors can affect the experiment (temperature, humidity, light, wind, etc.Experiments are usually not 100% accurate. This means that an experiment is most of the time, with error. Errors usually occur in measurement or observation and can cause them to be different from the true values of what is being measured.TYPES OF ERROR:
Error can either be systematic or random.
Random errors are errors causes by unpredictable circumstances in the experimentSystematic errors are caused by faulty or inaccurate instruments used.According to this question, the following can be sources of errors in an experiment:
People observe things slightly differently and can make mistakesMeasuring devices have limits, might be broken, or might be calibrated incorrectlyOutside factors can affect the experiment (temperature, humidity, light, wind, etc.Learn more: https://brainly.com/question/14149934
What causes stress in rocks? and What happens when stress is released?
Answers
Which choice is not true of a liquid in a glass capillary with a convex meniscus?a. The liquid has strong cohesive forces.b. The liquid level will be lower inside the capillary when a capillary is inserted into a bowl of the liquid.c. The liquid will have a convex meniscus as it moves in the capillary.d. The behavior of the liquid is driven by strong interactions with the capillary glass.
Answers
The correct answer is the liquid level will be lower inside the capillary when a capillary is inserted into a bowl of the liquid.
When a liquid is placed in a glass capillary with a convex meniscus, the behavior of the liquid is driven by strong cohesive forces between the liquid molecules and strong interactions with the capillary glass. As a result, the liquid will have a convex meniscus, meaning that the surface of the liquid will be curved outward. This is due to the forces acting on the liquid in the capillary. However, when a capillary is inserted into a bowl of the liquid, the liquid level inside the capillary will be higher than the liquid level outside the capillary. This is because the cohesive forces between the liquid molecules are stronger inside the capillary, where the liquid is in contact with the walls of the capillary, than they are outside the capillary. As a result, the liquid will be pulled up the walls of the capillary, causing the liquid level inside the capillary to be higher than the liquid level outside the capillary. This is opposite to what is stated in choice b, which is why it is the correct answer.
to know more about meniscus-
https://brainly.com/question/28013459
#SPJ4
Its a pick and HELP FASTS!!!!!

Answers
The cellular organization of human body starts from the cells itself and tissues then organ and organ systems. Here the the image in third box is cell or a tissue the first level and they joined together to form the second level as seen in the second box then heart and the human body.
What is organ system?Human body is made of various organ systems. Organ systems are a group of organs joined to perform one more functions. Different organ systems include digestive system, respiratory system, circulatory system etc.
The organ system including heart is circulatory system by which blood flow throughout the body. Cells are the basic level of this organization. Cells combines to form tissues asin the second image.
Tissue combines to form an organ as heart in first image and then the organ system and human body as seen in the last image.
To find more on organ system, refer here:
https://brainly.com/question/13278945
#SPJ1
how would you make up 100 ml of 50 mm tris-hcl buffer at ph 7.4 in a lab, using the 0.5m tris base stock solution, and any other required materials?
Answers
To make 100 ml of 50 mM Tris-HCl buffer at pH 7.4 in a lab, you can follow these steps:
Measure out the desired amount of Tris base stock solution, which has a concentration of 0.5 M. For 100 ml of 50 mM Tris-HCl buffer, you will need 50 mM / 0.5 M = 0.1 moles of Tris base.
Transfer the calculated amount of Tris base to a suitable container and add distilled water to make up the desired volume (100 ml in this case).
Adjust the pH of the solution to 7.4 using a suitable buffer such as HCl or NaOH. To do this, you can add a small amount of the acid or base at a time and measure the pH until you reach the desired value of 7.4.
Stir the solution until it reaches homogeneity and the pH is stable. You can measure the pH again to confirm that it is 7.4.
Store the Tris-HCl buffer at room temperature in a suitable container until it is ready to be used.
It is important to note that the pH of the buffer should be checked and adjusted if necessary before use, as pH drift may occur over time.
Learn more about buffer:
brainly.com/question/27959511
#SPJ4
the partial pressure of oxygen in the atmosphere is 0.2095. calculate the partial pressure in kpa. round answer to significant digits.
Answers
The value of pressure 7.0984 mmHg, the value of torr7.0984 torr. To determine the proportion of pressure from the total pressure that may be assigned to each individual gas, divide the mole fraction of each gas by the total mole fraction.
How can I determine the partial pressure?Calculating partial pressures can be done in one of two ways: 1) To determine each gas's unique pressure in a mixture, use PV = nRT. 2) .To determine the proportion of pressure from the total pressure that may be assigned to each individual gas, divide the mole fraction of each gas by the total mole fraction.Explanation:
Data obtained from the question include:
Partial Pressure of Ar = 0.2095 atm
1. The value of pressure in mmHg is obtained as shown below:
1 atm = 760mmHg
Therefore, 0.2095 atm = 0.2095 x 760 = 159.22mmHg
2. The value of the pressure in torr is obtained as shown below:
1 atm = 760torr
Therefore,0. atm = 0.2095 x 760 = 159.22torr.
To learn more about Partial pressures refer to:
https://brainly.com/question/15302032
#SPJ4
consider the equilibrium that exists for a saturated aqueous solution of pbcl2. pbcl2s⇄pb2 aq 2 cl-aq which expression gives the solubility product constant ksp for pbcl2 if the [pb2 ]
Answers
The solubility product is 4n³.
The equilibrium constant for a solid's dissolving into an aqueous solution is called the solubility product constant. It is represented as K(sp).
A solution is said to be saturated when it has dissolved all of the solutes it can. At a certain temperature, no additional solute can dissolve in a saturated solution.
Consider the equilibrium for a saturated PbCl₂ aqueous solution.
PbCl₂(s) ⇄⇄ Pb₂ (aq) + 2Cl⁻ (aq)
The equilibrium constant for the dissociation reaction of the PbCl₂ solution is:
K(sp) = [Pb²⁺] [Cl⁻]²
Now, [Pb²⁺] = n mol/L
In the solution, there is one-mole Pb²⁺ and two moles of Cl⁻ .
Therefore,
[Cl⁻] = 2n mol/L
So, the equilibrium constant for the dissociation reaction will be:
K(sp) = [Pb²⁺] [Cl⁻]²
K(sp) = n × (2n)²
K(sp) = n × 2n × 2n
K(sp) = 4n³
Learn more about equilibrium here:
https://brainly.com/question/517289
#SPJ4
basalt flowing out across miles of land
Answers
An eruption from a volcano can cause massive flows of basalt, a common kind of volcanic rock.
What brings about basalt flows?Due to the low viscosity of molten basalt lava (between 45% and 52%) and its low silica concentration, lava flows can spread over large areas quickly before cooling and solidifying.
Where are the basalt flows?One of the world's largest volcanic provinces is the flood basalt province known as the Deccan Traps, which is situated on the Deccan Plateau in west-central India. The Deccan Plateau, which spans about 500 000 km2, is made up of a series of flat-lying basalt lava flows that are more than 2000 m thick.
To know more about basalt visit:-
https://brainly.com/question/28268226
#SPJ1
a 25.00 gram sample of an unknown metal initially at 99.0 degrees celcius is added to 50.00 grams of water initially at 12.6 degrees celcius. the final temperature of the system is 20.15 degrees celcius. calculate the specific heat of the metal
Answers
The specific heat of the metal is 19.99 J / g·°C
Given,
The mass of the unknown metal = 25.00 gThe initial temperature of the unknown metal = 99.0° CThe mass of the water = 50.00 gThe initial temperature of the water = 12.6° CThe final temperature of the system (equilibrium temperature) = 20.15° CWe are required to find the specific heat of the metal.
Specific Heat Formula
We know that the specific heat of a substance is defined as the amount of heat energy required to raise the temperature of 1 gram of that substance by 1° C.
It is denoted by “C.”From the problem, the heat gained by the water will be equal to the heat lost by the metal. This can be represented by the equation below:
q gained by the water = q lost by the metal where, q = m * C * ∆T
where, m is the mass of the substance , C is the specific heat of the substance , ∆T is the change in temperature of the substance
The heat gained by the water can be calculated as:
q gained by the water = m * C * ∆T= 50.00 g * 4.184 J/g·°C * (20.15 - 12.6)°C= 50.00 g * 4.184 J/g·°C * 7.55°C= 1576.78 J
The heat lost by the metal can be calculated as:
q lost by the metal = m * C * ∆T= 25.00 g * C * (99.0 - 20.15)°C= 25.00 g * C * 78.85°C= 1971.25 C * g * °C
The two equations can be equated to get:
C * 78.85°C = 1576.78 JC = 1576.78 J / 78.85°C= 19.99 J / g·°C
Therefore, the specific heat of the unknown metal is 19.99 J / g·°C.
To know more about the specific heat visit https://brainly.com/question/11297584?
#SPJ11
WILL GIVE BRAINLIEST!!!
What is the mass of NaCl required to make 160 grams of a 32% solution of NaCl in water?
Answers
Answer:
19.20 g. Explanation: ∵ mass % = [mass of solute/mass of solution] x 100
matter is passed down from the living part of an ecosystem to the non living part when witch of the following take place ?
Answers
How many molecules are in 90g of AgNO3
Answers
Answer: There are 0.5298072502356179 molecules in 90g of AgNO3
Hope this helps!
How to balance this equation
NaCl +
F₂ →
NaF +
Cl₂
Answers
Answer:
The balanced equation is:
2NaCl + F₂ → 2NaF + Cl₂
Explanation:
Balancing an equation is a bit like playing musical chairs. There is no quick formula for success, but a little trial and error will lead to the solution. And a little practice goes a long way.
NaCl + F₂ → NaF + Cl₂
Check how many times an element appears on each side of the equation:
Element Reactants Products Balanced?
Na 1 1 Yes
Cl 1 2 No
F 2 1 No
The equation has two elements not balanced. Lets start with the Cl. Each molecule of Cl2 has 2 Cl atoms, but there is only 1 brought in by the NaCl. We can't use Cl as a product, since it does not exist under normal conditions. So lets make the coefficient for the NaCl 2, instead of 1.
2NaCl + F₂ → NaF + Cl₂
Element Reactants Products Balanced?
Na 2 1 No
Cl 2 2 Yes
F 2 1 No
Now the sodium is unbalanced due to this change. Add a coefficient of 2 to the NaF to accomodate the extra sodium atom:
2NaCl + F₂ → 2NaF + Cl₂
Element Reactants Products Balanced?
Na 2 2 Yes
Cl 2 2 Yes
F 2 2 Yes
The equation is now balanced. Each atom can be accounted for.
2NaCl + F₂ → 2NaF + Cl₂
====
This process may seem a bit tedius, but it becomes easier with practice: Some points that can help:
Use pencil and paper. Be prepared to try/erase the options.Look for the most "difficult" or complex moplecule first and assign a coefficent of 1 just to get started. Each molecule must be a whole number in the final equation.Although the final equation needs to have whole numbers, there is nothing wrong with fractions to start the process. For the example above, instead of doubling the NaCl, add a (1/2) as the Cl2 coefficient:NaCl + F₂ → NaF + (1/2)Cl₂
Element Reactants Products Balanced?
Na 1 1 Yes
Cl 1 2 Yes
F 2 1 No
Now do the same for the F2 on the reactant side:
NaCl + (1/2)F₂ → NaF + (1/2)Cl₂
Element Reactants Products Balanced?
Na 1 1 Yes
Cl 1 2 Yes
F 2 2 Yes
The equation is now balanced. But there is no such thng as 1/2 of a molecule (except, perhaps, in politics).
So multiply all the coefficients by a facor that makes them whole numbers. We can use 2, in this case.
2NaCl + 2(1/2)F₂ → 2NaF + 2(1/2)Cl₂
2NaCl + 1F₂ → 2NaF + 1Cl₂
This is the correct equation. The point is that a non-whole coefficient may be used to start, as long as they are all whole numbers at the conclusion.
Given 3. 82g of (NH4)2O find how many atoms of (NH4)2O
Answers
Answer: 4.41*10^23 atoms
Explanation:
3.82*(6.02*10^23)/52.10= 4.41*10^23 atoms
N*2=14.01*2=28.02
H*8= 1.01*8= 8.08
O*1=16.00*1=16.00
Add them together to get 52.10 g