Ctab, the surfactant used in this experiment does not dissolve in hexane. Why not?.
Answers
The surfactant does not dissolve in hexane due to its polar nature.
Why the surfactant does not dissolve?The surfactant used in this experiment does not dissolve in hexane because hexane is non-polar solvent whereas the surfactant is polar in nature and we know that polar solutes only dissolve in polar solvent so we can conclude that the surfactant does not dissolve in hexane due to its polar nature.
Learn more about hexane here: https://brainly.com/question/24574179
Related Questions
The cultures of prehistoric humans are known mostly through the excavation of stone tools and other relatively imperishable artifacts. The early tool making traditions are often referred to as being paleolithic (literally "Old Stone Age). The Oldowan and Acheulian tool traditions of the first humans were the simplest applied research basic research Scientihe thought O philosophies technologies
Answers
The cultures of prehistoric humans are primarily known through the excavation of stone tools and other durable artifacts, such as the Oldowan and Acheulian tool traditions.
Stone tools and imperishable artifacts serve as key archaeological evidence for understanding prehistoric cultures. Through meticulous excavation and analysis, archaeologists have been able to piece together the lifestyles, technological advancements, and social behaviors of early human societies. The term "paleolithic" refers to the Old Stone Age, a time when humans relied on stone tools as their primary implements.
The Oldowan tool tradition is considered the earliest stone tool industry, dating back around 2.6 million years ago. It is characterized by simple tools, such as choppers and scrapers, which were crafted by flaking off pieces from larger stones. These tools were primarily used for basic activities like butchering and processing animal carcasses.
Later, the Acheulian tool tradition emerged around 1.76 million years ago, representing an advancement in stone tool technology. Acheulian tools, such as handaxes and cleavers, were more refined and standardized, showcasing an increased level of sophistication in tool-making techniques. These tools served a wide range of purposes, including hunting, woodworking, and shaping raw materials.
By studying the Oldowan and Acheulian tool traditions, researchers gain valuable insights into the cognitive abilities, cultural development, and technological progress of early humans. The examination of these artifacts provides evidence of their adaptability, problem-solving skills, and the gradual refinement of their tool-making techniques over time.
Learn more about prehistoric humans
brainly.com/question/28301954
#SPJ11
phcl2 lewis structure
Answers
Answer:
What do you want answered?
Explanation:
Computer architecture. Can you
please answer all the question. True or false, Thank you
1. Indicate whether the following statements are true of false. ( ) In the same OSM, all pages must have the same size. ( ) In the same OSM, all segments must have the same size. ) Internal fragmentat
Answers
a. False. In the same OSM (Operating System Memory), it is not necessary for all pages to have the same size. Different pages can have varying sizes depending on the memory allocation requirements of the system.
b. False. In the same OSM, all segments do not need to have the same size.
Segments can have different sizes based on the specific needs of the programs or processes running in the system.
Segmentation allows for flexible memory allocation by dividing the memory into logical segments.
c. True. Internal fragmentation occurs when memory is divided into smaller areas to accommodate smaller data blocks, but the remaining memory within each block is too small to be utilized for another block.
As a result, these small unused portions of memory remain empty, leading to inefficient memory utilization.
This is a disadvantage of the paging architecture employed in a computer's memory management system. It can result in wasted memory space and lower overall system performance.
Read more about Operating System Memory
https://brainly.com/question/30593994
#SPJ11
What volume (in mL) of 0.2600 M HBr is
required to neutralize 40.00 mL of 0.8000 M
NaOH?
Answers
The volume of 0.26 M HBr required to neutralize 40 mL of 0.8 M NaOH is 123.08 mL
We'll begin by writing the balanced equation for the reaction. This is given below:
HBr + NaOH —> NaBr + H₂O
From the balanced equation above,
The mole ratio of the acid, HBr (nA) = 1
The mole ratio of the base, NaOH (nB) = 1
From the question given above, the following data were:
Volume of base, NaOH (Vb) = 40 mL
Molarity of base, NaOH (Mb) = 0.8 M
Molarity of acid, HBr (Ma) = 0.26 M
Volume of acid, HBr (Va) =?The volume of the acid, HBr needed for the reaction can be obtained as follow:
MaVa / MbVb = nA / nB
0.26 × Va / (0.8 × 40) = 1
0.26 × Va / 32 = 1
Cross multiply
0.26 × Va = 32
Divide both side by 0.26
Va = 32 / 0.26
Va = 123.08 mLTherefore, the volume the acid, HBr needed to neutralize the base, NaOH is 123.08 mL
Learn more: https://brainly.com/question/9659507
PLEASE ANYONE HELP!!!
How does an increase in -CH2 groups in an organic chain affect the boiling point?
A. The boiling point increases as more -CH2 groups are added.
B. The boiling point increases up to 4 CH2 groups and then begins to decrease.
C. The boiling point is not affected by -CH2 groups, only by functional groups.
D. The boiling point decreases as more -CH2 groups are added.
Answers
Answer:
I believe it's a.
Explanation:
Why does wood float besides it being less dense
Answers
Answer:
The only reason wood floats is because it is less dense and has big openings and gaps which allows air in.
Wood that sinks has very tiny openings. The ratio between weight and volume is called density. An object that is less dense than water can be held up by water, and so it floats.
Hope this helps! please mark me brainliest!
God bless :)
3.
Find the correct element based on the information given (on the right side) and then drag
the element over.
Cesium
Magnesium
Bismuth
Bromine
Period 3 & Group 2A
Answer by dragging items here
Period & Group 7A
Answer by dragging items here
Period 6 & Group 1A
Answer by dragging items here
Period 6 & Group SA
Answer by dragging items here
Answers
Period 3 & Group 2A = Magnesium
Period & Group 7A = Bromine
Period 6 & Group 1A = Cesium
Period 6 & Group SA = Bismuth
What is groups and period in periodic table ?Groups - The horizontal row of the periodic table that represents an element's valence electron count.
Periods - The horizontal columns of the periodic table that represent an element's number of electron shells. Families: Compounds with comparable characteristics and the same number of valence electrons.
Elements in the periodic table can be divided into groups and eras. The periodic table is organised into horizontal rows for periods and vertical columns for groupings. As you proceed over a period or down a group, the atomic number rises.Learn more about Periodic table here:
https://brainly.com/question/15987580
#SPJ13
A provider prescribes heparin 18 units/kg/hr by continuous IV infusion for a client who weighs 132 lb. Available is heparin 50 units in 1 mL of 0.9% NaCl. Determine how many mL/hr to administer. (Round the answer to the nearest tenth.)
Answers
A continuous IV infusion of the heparin solution should be given at a rate of around 21.6 mL/hr. To determine the needed mL/hr of heparin infusion: Converting the client's weight from pounds (lb) to kilogrammes is the first step (kg).
1 kilogramme (2.246 lb) 59.874 kg = 132 lb (rounded to three decimal places)
Step 2: Determine how many units of heparin are needed overall per hour.
Heparin dose: 59.874 kg x 18 units/kg/hr = 1077.732 units/hr (rounded to three decimal places)
Decide how much of the heparin solution to provide each hour in step three.
Total units needed / Heparin concentration = Heparin solution volume
Heparin solution volume = 1077.732 units per hour / 50 units per millilitre 21.55464 mL per hour (rounded to three decimal places)
Thus, a continuous IV infusion of the heparin solution should be given at a rate of around 21.6 mL/hr.
To know more about heparin solution:
https://brainly.com/question/31118361
#SPJ12
- Why do we learn the Bohr Model?
Answers
Answer:
The Bohr model shows the atom as a small, positively charged nucleus surrounded by orbiting electrons. The Bohr model is simpler and relatively easy to understand. So,we learn the Bohr Model.
which side of the periodic table are the metals on
Answers
Answer:
left
Explanation:
Metals are to the left of the line (excluding hydrogen, which is a nonmetal), nonmetals are to the right of the line, and metalloids are to the immediate right of the line.
(Hope this helps can I pls have brainlist (crown)☺️)

What property of a metal does the image represent
Answers
Answer:
malleable
Explanation:
The image represent in malleable property of metal.
The image possibly represents the photoelectric effect of a metal, which is when it emits electrons after being exposed to electromagnetic radiation. Metals are also characterized by physical properties such as conductivity, malleability, metallic luster, and metallic bonding.
Explanation:Based on your question, the image possibly represents the photoelectric effect, a key property of metals. This phenomenon occurs when a metal surface exposed to electromagnetic waves of a certain frequency absorbs radiation and emits electrons. These emitted electrons are called photoelectrons. Metals can also exhibit free electron model behavior, where electrons freely roam within the metal structure.
Metals possess unique physical properties like conductivity, malleability, and metallic luster. Malleability refers to the metal's ability to deform without breaking, while conductivity refers to the metal's ability to transfer heat or electricity. A metallic luster gives metals their characteristic shiny appearance.
Finally, metals are also known for their metallic bonding—a unique force that holds together the atoms within a metallic solid. Metallic bonding gives rise to many useful and varied bulk properties of metals.
Learn more about Properties of Metals here:https://brainly.com/question/33514448
#SPJ2
What is the mass in grams of a 10.2cm3 block of copper
Answers
Answer:
91.4
Explanation:
Givens
d = 8.96
v = 10.2 cm^3
m =?
Formula
d = m / V
Solution
8.96 = m / 10.2 Multiply both sides by 10.2
8.96 * 10.2 = m
m = 91.392 grams
m = 91.4 grams rounded.
Give one example of how environmental factor could influence the way a gene is expressed.
Answers
Answer:
The presence of drugs or chemicals in an organisms environment can also influence gene expression in the orgains.
(Look at the chart) You are trying to figure out the identity of a lump of yellow metal you found. You use a scale to determine that it has a mass of 50g.
You fill a container with 100mL.
What kind of metal is the lump you found?
LOOK AT THE CHART
-impossible to know
-gold
-copper
-silver

Answers
Explanation:
Volume of solid metal = 102.6ml - 100ml = 2.6ml.
Mass of solid metal = 50g.
Density = Mass / Volume = 50g / 2.6ml = 19.2g / cm³.
Also from our knowledge, only gold metal is yellowish here.
Hence the answer is gold metal.
A compound contains 40% calcium, 12% carbon, and 48% oxygen by mass. What is the empirical formula of this compound?
OCaco,
O CaC306
o Caco,
O CaC204
Answers
Answer:
CaCO3
Explanation:
E.F. is looking for the lowest possible ratio
Although ATP is the main energy currency in cells, other molecules, such as NAD, play a central role in some metabolic pathways by transferring electrons. The oxidized form of NAD is NAD+, and the reduced form is NADH. Identify the components of NAD+ and ATP. NH, O=P 0 NH, N OH OH O 0 NH 0 0 0 O=P-0 OH OH OH OH ATP NAD Answer Bank deoxyribose phosphate adenine nicotinamide ribose Select the components that are common to both ATP and NAD. ribose adenine deoxyribose phosphate nicotinamide
Answers
The components that are common to both ATP and NAD are: adenine and ribose. Adenine and ribose are both found in ATP and NAD molecules.
What are ATP and NAD?ATP stands for Adenosine Triphosphate, which is the primary energy carrier in cells. ATP is an energy-rich molecule that stores energy that can be used by the cell. It is composed of three phosphate groups, an adenine base, and a ribose sugar.
NAD stands for Nicotinamide Adenine Dinucleotide, which is an electron carrier molecule that is involved in many cellular metabolic reactions. It is composed of two nucleotides (adenine and ribose) linked by two phosphate groups.
The oxidized form of NAD is NAD+ while the reduced form is NADH.
The components of ATP and NAD are: Adenine and ribose are the two components common to both ATP and NAD.
Other components are specific to each molecule, as follows: ATP components: Three phosphate groups An adenine base A ribose sugar NAD+ components: Nicotinamide (a type of vitamin B3)Adenine A ribose sugar Two phosphate groups.
To learn more about "ATP" here:
https://brainly.com/question/30387542#
#SPJ11
formula for finding speed using binding energy and mass
Answers
Answer:
The formula for finding speed using binding energy and mass is E=mc2.
Which element has the following complete electron configuration : 1s22s22p63s23p64s23d5?
Answers
Answer:
Manganese
Explanation:
The electronic configuration of the atom is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵
The powers in this configuration is the number of electrons in each orbital:
2 + 2 + 6 + 2 + 6 + 2 + 5 = 25
The compound has 25 electrons and it is Manganese
The electron configuration 1s22s22p63s23p64s23d5 corresponds to the element manganese (Mn).
In the electron configuration provided:
1s2 represents the filling of the 1s subshell with two electrons.
2s2 represents the filling of the 2s subshell with two electrons.
2p6 represents the filling of the 2p subshell with six electrons.
3s2 represents the filling of the 3s subshell with two electrons.
3p6 represents the filling of the 3p subshell with six electrons.
4s2 represents the filling of the 4s subshell with two electrons.
3d5 represents the filling of the 3d subshell with five electrons.
Based on this electron configuration, we can correctly identify the element as manganese (Mn), which has an atomic number of 25.
Know more about electron configuration:
https://brainly.com/question/29157546
#SPJ6
An isotope has a half-life of 2 days. How much will be left after 6 days?
O 1/6
O 1/2
O 1/8
O 1/4
Answers
what is the change in internal energy (in j) of a system that absorbs 0.335 kj of heat from its surroundings and has 0.966 kcal of work done on it? give your answer in scientific notation.
Answers
The change in the internal energy is 4.395 kj
What is internal energy?
Rising temperature and changes in state as well as phase from solid state to liquid as well as liquid to gas cause an increase in internal energy. Planetary bodies could be viewed as a fusion of heat engines and heat reservoirs. Internal energy E is stored in the heat reservoirs, and some of it is converted into different kinds of mechanical, electrical, and chemical energies by the heat engines. The sum of the kinetic energy brought on by the molecule's movement and the potential energy brought on by the vibrational motion as well as electric energy of atoms inside of molecules makes up the internal energy U of the a system or a body with clearly defined boundaries. The energy contained in every chemical bond is also referred to as internal energy.
Given;
Q = +0.335 kj
W = - 0.966 kcal = -966 cal = - 4.04kj
del E int = +0.355 - [-4.04]
= 4.395 > 0
{Increase in internal energy}
To learn more about internal energy from the given link
https://brainly.com/question/25737117
#SPJ4
why is octet inactive
Answers
Answer:
Octet is inactive because it has a filled shell of electrons or the atom doesn't need to either gain electrons.
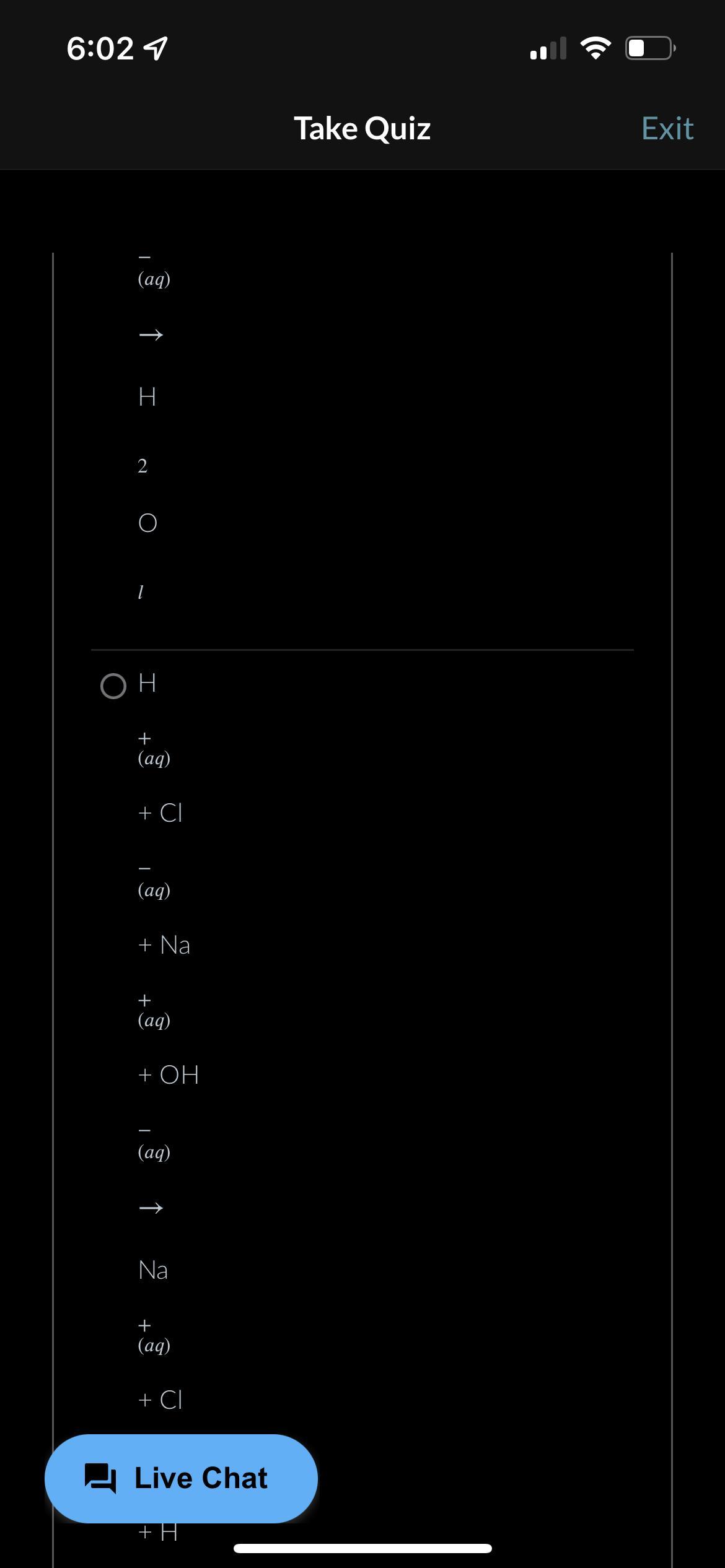
LOTS OF POINT PLS HELP!!!! What is the balanced, net ionic equation for the reaction shown below? HCI (aq) + NaOH (ag)-> NaCI (aq) +H2P(l)



Answers
The net ionic equation is written as H^+(aq) + OH^-(aq) -------> H2O(l)
What is the net ionic equation?The term net ionic equation refers to the equation that shows the ions that underwent a change in the reaction. We have to note that the reaction species here must be ionic species which are able to dissociate into ions in solutions.
Now the first step is to put down the molecular equation. The molecular equation shows the reaction of the compounds as follows;
HCI (aq) + NaOH (ag)-> NaCI (aq) +H2O(l)
Next, we put own the complete ionic reaction equation as follows;
H^+(aq) + Cl^-(aq) + Na^+(aq) + OH^-(aq) -------> Na^+(aq) + + Cl^-(aq) + H2O(l)
Next we have the net ionic equation;
H^+(aq) + OH^-(aq) -------> H2O(l)
Learn more about ionic reaction equation:https://brainly.com/question/21368817
#SPJ1
In the formation of ionic compounds a metal will ______ electrons to form an ion with the same number of electrons as the nearest ______ gas.
Answers
In the formation of ionic compounds, a metal will lose electrons to form an ion with the same number of electrons as the nearest noble gas.
Ionic compounds are formed through the transfer of electrons between a metal and a non-metal. Metals, which are located on the left side of the periodic table, tend to have few valence electrons. These valence electrons are loosely held and can be easily removed, resulting in the formation of positively charged ions, known as cations.
When a metal reacts with a non-metal, such as an element from the halogen group, the metal atom loses one or more electrons to attain a stable electron configuration. The number of electrons lost corresponds to the difference between the number of valence electrons in the metal atom and the number of electrons needed to achieve the electron configuration of the nearest noble gas.
Noble gases, found in Group 18 of the periodic table, possess a complete outer electron shell, making them highly stable and unreactive. By losing electrons, the metal ion achieves the same electron configuration as the noble gas nearest to it in the periodic table. This transfer of electrons from the metal to the non-metal results in the formation of an ionic bond, where the oppositely charged ions are attracted to each other, forming a crystal lattice structure.
In summary, metals lose electrons in the formation of ionic compounds to attain the electron configuration of the nearest noble gas, ensuring greater stability for the resulting ions.
Learn more about Electron configuration
brainly.com/question/13497372
#SPJ11
1.00 pint of milk has a volume of how many milliliters? ( 2 pints = 1 quart)
Answers
1.00 pint of milk is equal to 473.18 milliliters, based on the conversion factor of 1 pint = 473.18 milliliters.
To convert pints to milliliters, we can use the conversion factor of 1 pint = 473.18 milliliters.
Since we have 1.00 pints of milk, we can multiply it by the conversion factor to find the volume in milliliters:
1.00 pint * 473.18 milliliters/pint = 473.18 milliliters.
Therefore, 1.00 pint of milk is equivalent to 473.18 milliliters. It's important to note that this conversion factor is based on the standard definition of a pint, which is equal to 473.18 milliliters. In some countries, the pint may have a different value, so it's essential to use the appropriate conversion factor based on the specific context or region.
learn more about conversion here:
https://brainly.com/question/31389471
#SPJ11
how do we determine the number of neutrons an element has
Answers
Answer:
To determine the amount of neutrons an element has you would subtract the number of protons/atomic number from the atomic mass.
Explanation:
In which orbital would the valance electrons for carbon(C) be placed?
Answers
Carbon has an atomic number of 6 so its electron configuration will be 1s² 2s² 2p². It has two orbitals as indicated with the 2 as its period number with the outer orbital have 4 valence electrons. So carbon is in the p-orbital, period 2 and in group 4.
write the formula for the conjugate acid for each substance below. Please include states of matter.
F- (aq)= HF
Cl- (aq) = HCl
NH3 (aq) = NH4+
I have found the conjugate acids but not the states. how can I find the states?
Answers
To determine the states of matter for the conjugate acids, we can consider their common forms when dissolved in water.
F- (aq) -> HF (aq)
Cl- (aq) -> HCl (aq)
NH3 (aq) -> NH4+ (aq)
F- (aq) represents the fluoride ion in aqueous solution. Its conjugate acid is HF (aq), which stands for hydrofluoric acid. When dissolved in water, F- acts as a base and can accept a proton to form HF.
Cl- (aq) represents the chloride ion in aqueous solution. Its conjugate acid is HCl (aq), which stands for hydrochloric acid. When dissolved in water, Cl- acts as a base and can accept a proton to form HCl.
NH3 (aq) represents ammonia in aqueous solution. Its conjugate acid is NH4+ (aq), which stands for ammonium ion. Ammonia acts as a base and can accept a proton to form the ammonium ion, NH4+.
The states of matter for the conjugate acids are given as (aq), indicating that they are in aqueous solution when dissolved in water.
To learn more about conjugate acids , visit
brainly.com/question/12584785
#SPJ11
The number of protons plus the number of neutrons in an atom is equal to its
atomic number
mass number
nuclear model
energy level
Answers
Explanation:
mass number....................
A student conducts an experiment to determine how the temperature of water affects the time for sugar to dissolve. In each trial, the
student uses a different amount of water and a different temperature of water.
What is wrong with this experimental design?
Answers
The fact that the student used different amount of water (another independent variable) is wrong with the experimental design
WHAT ARE THE COMPONENTS OF AN EXPERIMENT?
An experiment aims at solving a scientific problem or answering a scientific question. An experiment should contain a variable being changed called INDEPENDENT VARIABLE and a variable being measured called DEPENDENT VARIABLE. In an ideal experiment, only one independent variable should be used while every other variable should be kept constant. This is done so as not to affect the result of the experiment.In the experiment conducted by the student in this question, two independent variables were used i.e. the different amount of water and the different temperatures. This is what is wrong about the experimental design.
In a nutshell, the fact that two independent variables were used by the student is what is wrong about the experimental design.Learn more at: https://brainly.com/question/967776
Answer: In an ideal experiment, only one independent variable should be used while every other variable should be kept constant.
Consider the following reaction, which occurs at standard state:
2CI(g) -- CI2(g) H= -243.4 kJ
If 15.0g of CI is concerted to chlorine, determine the heat released from this reaction.
Answers
Answer:
The heat energy released from the reaction is approximately 51.4791 kJ
Explanation:
The given parameters are;
The heat of the reaction of 2Cl (g) → Cl₂ (g) = -243.4 J
The given mass of Cl being converted to chlorine gas, Cl₂ = 15.0 g
From the reaction for the heat of formation, we have;
2 moles of chlorine produces 1 mole of Cl₂ to release 243.4 kJ of energy per mole of Cl₂
The mass of 1 mole of Cl ≈ 35.453 g
Therefore, from number of moles, n = Mass/(Molar Mass), we have;
The number of moles of chlorine, in 15.0 g of chlorine = 15.0/35.453 ≈ 0.423 moles
Therefore, given that 2 moles of Cl produces 1 mole of Cl₂, we have;
0.423 moles of Cl will produce 0.423/2 ≈ 0.2115 moles of Cl₂
The heat energy released from the reaction is therefore, H ≈ 0.2115 × 243.4 kJ = 51.4791 kJ.