Cu(s) + 4 HNO3 (aq) --> Cu(NO3)2 (aq) + 2NO2 (g) + 2H2O(l)
Each student in a class placed a 2.00g sample of a mixture of Cu and Al in a beaker and placed the beaker in a fume hood. The students slowly poured 15.0mL of 15.8M HNO3(aq) into their beakers. The reaction between the copper in the mixture and the HNO3(aq) is represented by the equation above. The students observed that a brown gas was released from the beakers and that the solutions turned blue, indicating the formation of Cu2 (aq). The solutions were then diluted with distilled water to known volumes. Which of the following is true about the reaction?
A)16%.
B) 32%.
C) 64%.
D) 96%.
Answers
The percentage of copper (Cu) in the original mixture, calculated from the given data, is 188%. None of the given options (A, B, C, or D) accurately represent the calculated percentage.
To determine the percentage of copper (Cu) in the original mixture, we can use stoichiometry and the concept of limiting reactants.
From the balanced chemical equation:
1 mole of Cu reacts with 4 moles of HNO3 to form 1 mole of Cu(NO3)2.
Given that the students added 15.0 mL of 15.8 M HNO3, we can calculate the number of moles of HNO3 added:
moles of HNO3 = (15.0 mL) * (0.0158 mol/mL) = 0.237 mol HNO3
Since the stoichiometric ratio between Cu and HNO3 is 1:4, we need four times the moles of HNO3 for a complete reaction with Cu. Therefore, the number of moles of Cu in the original mixture can be calculated as:
moles of Cu = 0.237 mol HNO3 * (1 mol Cu / 4 mol HNO3) = 0.05925 mol Cu
Now we can calculate the mass of Cu in the original mixture:
mass of Cu = moles of Cu * molar mass of Cu
mass of Cu = 0.05925 mol * 63.55 g/mol = 3.76 g Cu
Finally, we can calculate the percentage of Cu in the original mixture:
percentage of Cu = (mass of Cu / total mass of the mixture) * 100
percentage of Cu = (3.76 g / 2.00 g) * 100 = 188%
To read more about copper, visit:
https://brainly.com/question/26449005
#SPJ11
Related Questions
A certain first-order reaction a → b is 25% complete in 42 min at 25°c. What is the half-life of the reaction?.
Answers
The half-life of the reaction is 101.9 min
First order reactionWhen a reaction's pace and reactant concentration are inversely correlated, the process is known as a first-order reaction. To put it another way, the reaction rate doubles when the concentration does. One or two reactants can be present in a first-order reaction, as in the case of the decomposition process.
The half-life of first-order reaction:-The amount of time it takes for the initial concentration of the reactant(s) to decrease by half is known as the half-life of a chemical reaction (abbreviated as "t1/2").
Calculation:-a→b
25% reacted means 75% remains
t=42 min
Rate constant
k=(2.303/t)(log a/a-x)
k=(2.303/42)(log 100/100-25 )
k=(0.054) (log 100/75)
k=(0.054)(0.1249)
k=0.0068 per min
half life
t1/2=(0.693/k)
=(0.693/0.0068)
=101.9 min
Learn more about first order reaction here :-
https://brainly.com/question/27754430
#SPJ4
What do we call a substance composed of atoms of more than one element that are held together by chemical bonds?
Compound
Crystal
Salt
Ion
Answers
A substance composed of atoms of more than one element that are held together by chemical bonds is called a compound. Therefore the correct option is option A.
A compound is a pure material that is created by chemically combining two or more distinct components in a specific order. Chemical bonds, which can be ionic or covalent, hold the atoms of a substance together.
The characteristics of compounds are distinct from the characteristics of the constituent parts.
For instance, sodium is a soft metal and chlorine is a greenish-yellow gas; nevertheless, when these two elements combine to produce sodium chloride (table salt), they create a white crystalline solid that is far more stable than the constituent parts of each element alone. Therefore the correct option is option A.
For such more question on chemical bond:
https://brainly.com/question/819068
#SPJ11
As the psub(K,a) of a series of weak acids increases, the strength of their corresponding conjugate bases will
Answers
For a series of weak acids, the increase in pKa means that the conjugate bases become more stable.
What is pKa?The term pKa refers to the negative logarithm of the acid dissociation constant. This is a constant that shows the extent to which an acid is dissociated in solution.
As the pKa of a series of weak acids increases, the conjugate bases become more stable.
Learn more about pKa:https://brainly.com/question/13178964
In the addition reaction of HBr to 3-methyl-3-hexene, what is the second step? a) formation of a carbocation at carbon three b) attack of bromide ion on carbon four c) attack of bromide ion on carbon three d) formation of a carbocation at carbon four
Answers
option (c) - attack of bromide ion on carbon three
The addition of HBr to 3-methyl-3-hexene proceeds via a Markovnikov addition mechanism, where the hydrogen (H) atom of HBr adds to the carbon atom with the most hydrogen atoms, and the bromide (Br) ion adds to the carbon with the least hydrogen atoms. The reaction proceeds in two steps:
The H atom of HBr adds to the double bond to form a carbocation intermediate. The addition of H+ leads to the formation of a secondary carbocation, which can form at either carbon 3 or carbon 4.
The Br- ion then attacks the carbocation to form the final product. The bromide ion will preferentially attack the carbocation at carbon 3, as it is more stable than the carbocation at carbon 4.
Therefore, the answer is option (c) - attack of bromide ion on carbon three.
The mechanism for the addition of HBr to 3-methyl-3-hexene is as follows:
Step-1
CH3 H
| |
CH3CH2C(CH3)CH=CH2 + HBr --> CH3CH2C(CH3)CH(H)CH2Br+
carbocation intermediate forms here
Step 2
CH3 H Br
| | |
CH3CH2C(CH3)CH(H)CH2Br+ --> CH3CH2C(CH3)CH(Br)CH2CH3
final product forms here
To know more about addition reaction:
https://brainly.com/question/22233874
#SPJ11
Why was Niels Bohr atomic model superior to all the earlier models
Answers
Answer:
because it showed how the electron could orbit the nucleus without falling into it.
i have added 15 l of air to a balloon at sea level (1.0 atm). if i take the balloon with me to denver, where the air pressure is 0.85 atm, what will the new volume of the balloon be at the same temperature?
Answers
The new volume of the ballon at the same temperature is 17.65litres.
What is Boyles Law?
Boyles Law states that the product of pressure and volume is constant until the temperature remains constant.
PV = constant defines the Boyles law.
As given,
V₁ = 15L, P₁ = 1.0atm, P₂= 0.85atm
P₁V₁ = P₂V₂
Substitute values respectively,
1 × 15 = 0.85 × V₂
V₂ = 15/0.85
V₂ = 17.65L
Hence, the new volume of the balloon at the same temperature is 17.65L.
To learn more about The Boyles law from the given link.
https://brainly.com/question/1696010
#SPJ4
iron (ii) hydroxide + sodium nitrate. Name the precipitate formed in reaction
Answers
A olvent i found to be 50. 0% oxygen, 37. 5% carbon, and 12. 5% hydrogen. What i the empirical formula of thi olvent
Answers
The empirical formula of the solvent is CH4.
Relative number of atoms
Of H= 25/1 = 25
Of C= 75/12 = 6.25
What is a solvent?
Solvents are a heterogeneous group of structurally different chemicals that can be used to dilute, dissolve, or disperse other compounds. The ability of a solvent to dissolve another molecule depends on the molecular structure and physical properties of both the solvent and the solute. Solvents can be categorized as organic or inorganic and in terms of chemical polarity. Polar solvents include water, alcohols, and other chemicals containing –OH, such as acetic acid, which have the ability to donate H+ and form hydrogen bonds. Polar solvents lacking the –OH group, including acetonitrile, dimethylformamide, and dimethylsulfoxide, are protophilic solvents and are used to dissolve less polar solutes.To know more about solvents and solutes, click the link given below:
https://brainly.com/question/17061863
#SPJ4
can you please help me with this
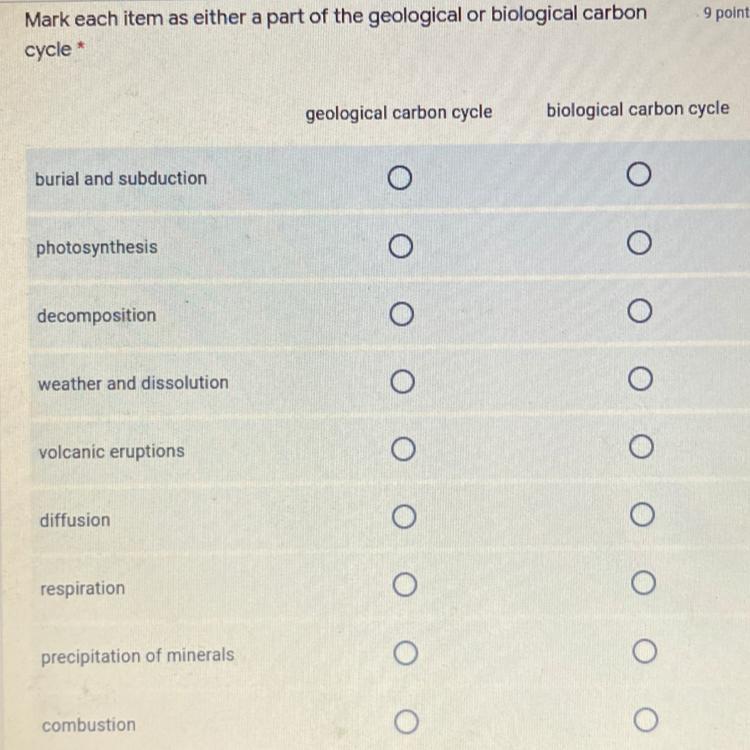
Answers
Explanation:
TUU-PQVH-MFG LINK for them who truely interested in Paranormal and have any doubt on Paranormal
True or False. Single covalent bonds are stronger than triple covalent
bonds
Answers
Answer:
False
Explanation:
Triple covalent bonds are much more stronger than single covalent bonds. In single bond, 2 electrons are shared, in double bond four electrons are shared and in triple bond six electrons are shared. Thus, triple bond is difficult to break since it is the strongest bond. Between the two atoms, stronger the bond, more stable the molecule. Thus, triple bond is more stable.
When an earthquake occurs, the speed of the seismic waves changes as they...
Answers
Answer:
heat up i think
Explanation:
Which of the following is a strong base? Select the correct answer below: O Nitrite ion O Sulfate ion O Cyanide ion Calcium hydroxide
Answers
Because its conjugate acid is weaker than other conjugate acids, the cyanide ion is a strong base.
C is the correct answer.
The cyanide ion is a good nucleophile as well. Therefore, depending on the solvent and alkyl group, a mixture of products must form in the reaction of alkyl halides with KCN.
Strong bases are those that are entirely dissociated in water. One or more hydroxide ions (OH-) are produced per base molecule when these chemicals ionise in water. In contrast, a weak base in water only partially splits into its ions.
A strong base is a substance with the capacity to eliminate a proton from a very weak acid. Or, when submerged in water, they totally separate into its ions. Sodium hydroxide and potassium hydroxide are two examples.
Learn more about Strong base:
https://brainly.com/question/27089588
#SPJ4
The complete question is:
Which of the following is a strong base? Select the correct answer below: A. Nitrite ion
B. Sulfate ion
C. Cyanide ion
D. Calcium hydroxide
The formula for barium chloride is bacl2. The chemical formula for barium chloride shows the number of of each element in the representative unit of the substance.
Answers
Yes, that's correct. The chemical formula "\(BaCl_2\)" represents the ratio of the elements in the compound barium chloride. In this case, it means there is one barium (Ba) atom and two chlorine (Cl) atoms in each representative unit of the compound.
What is the chemical formula?
The term chemical formula has to do with the way that the atoms that compose the substance are arranged. The arrangement of the atoms in the chemical substance would help us to have an idea of how the compound appears.
Now we are looking at the compound barium chloride of which we know that it is an ionic subsnace and since the substance is ionic in nature, we would talk about a formula unit of the compound as beings its representative unit in the crystal lattice of the substance.
The question above is incomplete, the complete question is:
The formula for barium chloride is BaCl2. The chemical formula for barium chloride shows the number of ___________ of each element in the ___________ representative unit of the substance.
Here you can learn more about the chemical formula
brainly.com/question/29519054
#SPJ4
Iron reacts with Oxygen gas to form Iron II Oxide according to the reaction
below.
4 Fe
+ 3 02
2 Fe2O3
How many moles of Iron II Oxide can be produced from 347.7 L of Oxygen gas at a temperature
of 74.3 °C and a pressure of 294.5 kPa?
Round Answers to 0.01 decimals

Answers
Answer:
2.0
Explanation:
a solution is prepared by dissolving 516.5 mg of oxalic acid to make 100.0 ml of a solution. a 10.00 ml portion is then diluted to 250.0 ml. what is the molarity of the final solution
Answers
The molarity of the final solution by dissolving 516.5 mg of oxalic acid to make 100.0 ml of a solution is 002295M.
Molarity of a given result is defined as the total number of intelligencers of solute per litre of result. The molality of a result is dependent on the changes in physical parcels of the system similar as pressure and temperature as unlike mass, the volume of the system changes with the change in physical conditions of the system.
516.5 mg of oxalic acid
516.5 mg = 0.5165 g
Number of moles,
= 0.5165 g *(1mole)/(90g)
= 0.0057388
Molarity for the solution,
= 0.0057388moles/.1liters
= 0.05738 M
The molarity of the final solution,
= (0.057388 moles/liter)*(10ml/250ml)
= 0.002295 M
Therefore, The molarity of the final solution by dissolving 516.5 mg of oxalic acid to make 100.0 ml of a solution is 002295M.
Learn more about Morality:
https://brainly.com/question/14757420
#SPJ4
A 0.520 g sample of an unknown nonelectrolyte compound is dissolved in 4.62 g of lauric acid (Kf = 3.90 .C/m).
The freezing point depression is determine to be 4.20 C. What is the molar mass of the compound?
Answers
Using the given mass of the compound (0.520 g) and the calculated moles, we can determine the molar mass of the compound.
To find the molar mass of the compound, we can use the formula:
ΔT = Kf * m
where ΔT is the freezing point depression, Kf is the cryoscopic constant (in this case, 3.90 °C/m), and m is the molality of the solution.
First, we need to calculate the molality (m) of the solution:
m = moles of solute / mass of solvent (in kg)
The mass of the solvent (lauric acid) is given as 4.62 g. Since the unknown compound is a solute, we need to convert its mass to moles:
moles = mass / molar mass
Given that the mass of the unknown compound is 0.520 g, we can now calculate the moles of the compound.
Next, we convert the mass of the solvent to kg by dividing by 1000:
mass of solvent (lauric acid) = 4.62 g / 1000 = 0.00462 kg
Now we can calculate the molality:
m = moles of solute / mass of solvent = (moles of the compound) / (mass of solvent)
Finally, we can use the freezing point depression formula to find the molar mass of the compound:
ΔT = Kf * m
Substituting the given values:
4.20 °C = 3.90 °C/m * m
Now solve for m:
m = (4.20 °C) / (3.90 °C/m)
Once we have the molality, we can calculate the moles of the compound:
moles = molality * mass of solvent (in kg)
Finally, we calculate the molar mass:
molar mass = mass of solute / moles of solute
Learn more about molar mass here :-
https://brainly.com/question/31545539
#SPJ11
a hallmark of many ionic compounds is their ability to dissolve in water through a process called
Answers
Ionic compounds exhibit a characteristic property of dissolving in water, a process known as aqueous dissolution.
Ionic compounds consist of positively charged cations and negatively charged anions. When placed in water, the polar nature of water molecules allows them to surround and separate the ions, breaking the ionic bonds within the solid lattice. This process is called aqueous dissolution or hydration. Water molecules form electrostatic interactions with the ions, creating a hydration shell around each ion.
This phenomenon occurs because water molecules have a partially negative oxygen atom and partially positive hydrogen atoms, enabling them to attract and stabilize the charged ions. The ability of ionic compounds to dissolve in water is essential for many chemical and biological processes, including solubility, electrolysis, and transportation of ions in biological systems.
For more information on hydration visit: brainly.com/question/10665473
#SPJ11
Given the system at equilibrium:
H3PO4 + 3 H2O <-----> 3 H3O+ + PO4^3-
If Na3PO4(s) is added, there will be a decrease in the
concentration of
A) Na+
B) PO4^3–
C) H3O+
D) H2O
Answers
Answer:
Adding Na3PO4(s) will introduce more PO4^3- ions into the solution, which will react with H3O+ ions to form more H3PO4 and H2O through the reverse reaction. This will shift the equilibrium to the left, decreasing the concentration of H3O+ ions and increasing the concentration of H3PO4 and H2O. Therefore, the concentration of H3O+ ions will decrease, and the correct answer is (C) H3O+.
50g of water were heated to boiling point at constant rate of 2.28KJ/minute.after boiling for 10minutes, the mass of water decreased to 40g calculate the heat of vaporization of water
Answers
Answer:
10g of water was vaporized by the 2.28K/J

Someone pls help me :( tysm if u do

Answers
Density is mass/volume.
Let's find the volume.
Volume = lwh = (2)(2)(2) = 8
The volume is 8 and the mass is 16.
D = m/v
D = 16/8
D = 2
2 grams per cm^3
State with reasons, whether sulphur dioxide is acting as an oxidizing agent or a reducing agent in each of the following reactions:
•2H2S(g) + SO2(g) -> 2H2O(l) + 3S(s)
•SO2(g) +H2O(l) +NaClO(aq) -> NaCl(aq) + H2SO4(aq)
Answers
Answer:
A) oxidizing agent is SO2
B) NaClO is the oxidizing agent
Explanation:
A) This is a redox reaction in which oxidation and reduction occur simultaneously.
Thus, in 2H2S(g) + SO2(g) -> 2H2O(l) + 3S(s);
H2S is reduced as follows;
H2S → S + 2H+ + 2e−
We can see that SO2 has been reduced while H2S gets oxidized since it has changed state from - 2 to 0 . Thus sulphur dioxide is the oxidizing agent.
B) SO2(g) + H2O(l) + NaClO(aq) -> NaCl(aq) + H2SO4(aq)
In this, SO2 undergoes oxidation and NaClO is the oxidizing agent
predict the missing component in the nuclear equation

Answers
The answer is C since both charge and mass have to be balanced on both sides of the equation.
5 points
What is the name of the atom pictured here? Use a periodic table if
needed. *
Answers
Answer:
There is nothing attached to this question so I unfortunately cannot help you
Explanation:
Which ionic compound would you predict to have a higher boiling point: NaBr or
Na2S
Why did you select this compound?
Answers
Answer:
The boiling point of NaBr is higher than that of Na2S
Explanation:
Bromine has higher electronegativity as compared to the sulphur. The ionic strength of the bond formed with Sodium is higher in the NaBr compound. The higher the ionic bond strength the higher will be the boiling point
Also the size of bromine is large as compared to that of sulphur. Large molecules consists of more electron and hence they create der Waals attractive forces due to which the boiling point of compound increases.
Hence, the boiling point of NaBr is higher than that of Na2S
At 2000 k the partial pressures of an equilibrium mixture of H2S, H2, and S are 0.0025, 0.041, and 0.035 atm, respectively. Calculate the value of the equilibrium constant Kp at 2000 K
H2 s(g) ⇌ H2(g)+ S(g)
Answers
At a temperature of 2000 K, the equilibrium constant Kp for the reaction H₂S(g) ⇌ H₂(g) + S(g) is approximately 1.47, based on the given partial pressures of the gases in the equilibrium mixture.
To calculate the value of the equilibrium constant Kp at 2000 K, we can use the given partial pressures of the gases in the equilibrium mixture.
The balanced equation for the equilibrium reaction is:
H₂S(g) ⇌ H₂(g) + S(g)
The expression for Kp is given by the partial pressures of the products raised to their stoichiometric coefficients divided by the partial pressure of the reactant raised to its stoichiometric coefficient.
Kp = (PH₂ * PS) / PH₂S
Where:
PH₂, PS, and PH₂S are the partial pressures of H₂, S, and H₂S, respectively.
Substituting the given values into the equation:
Kp = (0.041 * 0.035) / 0.0025
Calculating the expression:
Kp = 1.47
Therefore, the value of the equilibrium constant Kp at 2000 K is approximately 1.47.
To know more about the equilibrium constant refer here :
https://brainly.com/question/31321186#
#SPJ11
two piston-cylinder systems contain ideal gases at the same temperature and pressure. the gases are compressed in both systems to 10 bar, one isothermally and the other adiabatically. which system has the higher temperature at the end of the compression?
Answers
The adiabatically compressed system has the higher temperature at the end of the compression.
This is because, according to the ideal gas law, pressure and temperature are related by the equation:
PV^γ = constant
Where γ is the ratio of specific heat for the gas. Since the compression is adiabatic, no heat is added to the system and thus γ = 1.4 for most gases. Therefore, the pressure and temperature are related by:
\(\frac{P1}{T1} = \frac{P2}{T2}\)
Since P1 and P2 are equal (10 bar) but T2 is greater than T1, it follows that T2 must be greater than T1. Therefore, the adiabatically compressed system has a higher temperature than the isothermally compressed system.
learn more about specific heat Refer:brainly.com/question/11297584
#SPJ4
Kim dissolves 70.13 g of solid sodium chloride (NaCl) in enough distilled water to make 400 mL of stock solution. What volumes of stock solution and distilled water (DI) are needed to make a 150 mL solution of 1.2 M NaCl?
Answers
This problem is providing us with the mass of solid sodium chloride and the volume of water it was dissolved in. Thus, after diluting it, the volume of the stock solution was required and found to be 60. mL according to:
Diluted solutionsIn chemistry, when we are given a stock solution with specified volume and concentration, one is able to dilute it in order to use it for a specific purpose. This, by holding the number of moles constant, one can write:
\(C_1V_1=C_2V_2\)
Where the subscript 1 stands for the stock solution and 2 for the diluted one. Thus, one first calculate the initial concentration with the mass and volume:
\(M=\frac{70.13g/(58.44 g/mol)}{0.400L}=3.00M\)
Next, we solve for the volume of the stock solution, V1, as follows:
\(V_1=\frac{C_2V_2}{C_1}\)
Finally, we plug in the given data to obtain the result:
\(V_1=\frac{1.2M*150mL}{3.00M}\\ \\V_1=60.mL\)
Learn more about diluted solutions: https://brainly.com/question/26005640
How many grams of Cu(OH)2 will precipitate when excess KOH solution is added to 61.0 mL of 0.521 M Cu(NO3)2 solution?
Answers
27.7 g of Cu(OH)₂ will precipitate when excess KOH solution is added to 61.0 mL of 0.521 M Cu(NO₃)₂ solution.
The balanced chemical equation for the reaction is: Cu(NO₃)₂ + 2KOH → Cu(OH)₂↓ + 2KNO₃. Given, volume of Cu(NO₃)₂ solution = 61.0 mL = 0.0610 L. Concentration of Cu(NO₃)₂ solution = 0.521 M. To find the number of moles of Cu(NO3)₂, we use the formula: moles = concentration × volume (in L)moles of Cu(NO3)₂ = 0.521 × 0.0610 = 0.0318 mol.
From the balanced equation, we see that 1 mole of Cu(NO₃)₂ reacts with 2 moles of KOH to form 1 mole of Cu(OH)₂. So, the number of moles of KOH required for complete reaction = 2 × moles of Cu(NO₃)₂= 2 × 0.0318= 0.0636 mol. The molar mass of Cu(OH)₂ is 97.56 g/mol.
Mass of Cu(OH)₂ formed = number of moles of Cu(OH)₂ × molar mass of Cu(OH)₂= 0.0318 × 97.56= 3.10 g. So, 27.7 g of Cu(OH)₂ will precipitate when excess KOH solution is added to 61.0 mL of 0.521 M Cu(NO3)₂ solution.
Learn more about moles here:
https://brainly.com/question/15209553
#SPJ11
What is water's density at 91 ∘c? assume a constant coefficient of volume expansion.
Answers
Answer:
982.5 kg/m³
Explanation:
When the temperature of a fluid increases, it dilates, and because of the variation of the volume, it's density will vary too. The density can be calculated by the expression:
ρ₁ = ρ₀/(1 + β*(t₁ - t₀))
Where ρ₁ is the final density, ρ₀ the initial density, β is the constant coefficient of volume expansion, t₁ the final temperature, and t₀ the initial temperature.
At t₀ = 4°C, the water desity is ρ₀ = 1,000 kg/m³. The value of the constant for water is β = 0.0002 m³/m³ °C, so, for t₁ = 93°C
ρ₁ = 1,000/(1 + 0.0002*(93 - 4))
ρ₁ = 1,000/(1+ 0.0178)
ρ₁ = 982.5 kg/m³
Uniformitarianism is the idea that
Answers
Answer:
Uniformitarianism is the idea that the natural processes operating today are the same as the natural processes that operated in the past