Describe how you would find out if: a) milk contains protein. b) cheese contains fat.
Answers
a)To determine if milk contains protein, you can conduct a simple protein test. b)For testing the presence of fat in cheese, you can perform a simple lipid test.
To determine if milk contains protein, you can conduct a simple protein test. First, obtain a small sample of milk. Add a few drops of biuret reagent to the sample and observe any color change. If the sample turns violet or purple, it indicates the presence of protein in the milk.
For testing the presence of fat in cheese, you can perform a simple lipid test. First, acquire a small piece of cheese. Crush the cheese and place it in a test tube. Add a few drops of Sudan III solution to the test tube and shake it gently. If the mixture turns red or pink, it suggests the presence of fat in the cheese.
Both tests can provide initial indications of protein in milk and fat in cheese. However, for more accurate and quantitative results, additional laboratory methods, such as the Kjeldahl method for protein analysis and the Soxhlet extraction method for fat analysis, are often employed.
To know more about protein visit:
https://brainly.com/question/31017225
#SPJ11
Related Questions
which substance does not have a definite shape, color, or texture?
Answers
I believe gases do not have a definite shape, color, or texture.
A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape
So the answer is a gas
constraints of a crane
Answers
Answer:
The crane should have an electromagnet attached to its arm. The electromagnet: should have a soft iron core made from a bundle of short lengths of iron wire, must be strong enough to pick up several steel paperclips, nails or coins. Work on your own. This task will be assessed.
Explanation:
stable atoms have ____ paid of valence electrons
Answers
Answer: 8 electrons
Explanation:
Answer: 8 valence electrons
Explanation: This is because the maximum electron an energy level can hold is 8 making it a stable atom
What is the best description of chromosomes by the end of anaphase II of meiosis?
A) The chromosomes coil and become more compact.
B) The chromosomes line up in the middle of the cell.
C) The chromosomes split apart at the centromere.
D) The chromosomes reach opposite sides of the cell, incorporating into two haploid nuclei.
WILL GIVE BRAINLIEST**
** PROVIDE A CLEAR, EASY-TO-READ ANSWER**
Answers
The best description of chromosomes by the end of anaphase II of meiosis is option D) The chromosomes reach opposite sides of the cell, incorporating into two haploid nuclei.
What happens to chromosomes by the end of anaphase II in meiosis?
During anaphase II of meiosis, the sister chromatids of each chromosome are separated and pulled towards opposite poles of the cell. At the end of anaphase II, the chromosomes reach opposite sides of the cell, where they become incorporated into two separate haploid nuclei. The other options mentioned describe the events that occur during earlier stages of meiosis or during mitosis.
To learn more about chromosomes, visit: https://brainly.com/question/29393001
#SPJ1
Classify the reaction as endothermic or exothermique
Answers
When temperature of a reaction mixture rises as the energy is released during an exothermic process. Inside an endothermic reaction, its temperature drops as energy is absorbed.
What is an example of endothermic?Enthesogenic responses Heat is taken in. 1) Photosynthesis: In order to transform carbon water and carbon dioxide and oxygen, plants must absorb heat sunlight energy. 2) Heating an egg: The egg is cooked by absorbing heat energy from the pan.
What happens in endothermic reaction?Endothermic relates to any chemical reaction that absorbs heat from the environment. This energy that has been absorbed provides the activation energy for the process. This type of reaction is distinguished by its frigid feeling.
To know more about endothermic visit:
brainly.com/question/1371775
#SPJ1
An oven takes in 1200J of energy
and transfers 375J as useful energy.
Caculate the energy.
Answers
The energy is 375 J.
Energy is the quantitative belongings that are transferred to a frame or to a physical gadget, recognizable within the overall performance of labor and within the shape of warmth and mild. power is a conserved amount—the law of conservation of strength states that power may be converted in form, but now not created or destroyed.
Calculation:-
Total energy = 1200 J
Transfered emergy = 375 J
The useful energy is the energy to do work = 375 J.
The ability or strength to do paintings, along with the potential to transport an item (of a given mass) by means of the application of pressure. power can exist in a spread of forms, which includes electric, mechanical, chemical, thermal, or nuclear, and may be transformed from one form to another.
Learn more about energy here:-https://brainly.com/question/13881533
#SPJ1
Avogadro's number represents the number of units in a mole. What is this value?
o
3.01 x 1023
o 6.02 x 1023
1.20 x 1024
3.60 x 1024
Answers
The value of Avogadro's number is:
\(N = 6.02214076*10^{23}\)
So the correct option is the second one.
What is the value of Avogadro's number?Avogadro's number represents the number of molecules in a mole.
The value of the Avogadro's number is such that the mass of one mole of a compound is numerically equal to the mass of that compound in daltons.
And, by definition, Avogadro's number is:
\(N = 6.02214076*10^{23}\)
With this in mind, we conclude that the correct option is the second one.
If you want to learn more about Avogadro's number:
https://brainly.com/question/16060223
#SPJ1
Answer:
BExplanation:
6.02x10^23
On may 1, shilling company sold merchandise in the amount of $5,800 to anders, with credit terms of 2/10, n/30. The cost of the items sold is $4,000. Shilling uses the perpetual inventory system and the gross method. The journal entry or entries that shilling will make on may 1 is (are): on may 1, shilling company sold merchandise in the amount of $5,800 to anders, with credit terms of 2/10, n/30. The cost of the items sold is $4,000. Shilling uses the perpetual inventory system and the gross method. The journal entry or entries that shilling will make on may 1 is (are):.
Answers
The journal entry or entries that shilling will make on may 1 is (are): Accounts receivable 5,800
Journal entries are used by a bookkeeper to keep track of all the changes that a transaction can bring about in a company.Simple Journal Entries: In this case, only 2 accounts—one that is debited and the other that is credited—are impacted. 2. Compound or Combined Journal Entries: In this case, more than two accounts are impacted.In your accounting records, a journal entry acts as a record of a business transaction. In double-entry accounting, each transaction necessitates at least two journal entries.To know more about journal entry here
https://brainly.com/question/28390337
#SPJ4
what is 0.1 n hcl standardization?
Answers
The abbreviation 0.1 N HCl means normal hydrochloric acid.By comparing a solution to a standard solution with a known concentration, standardisation is the process of determining the precise concentration
By comparing a solution to a standard solution with a known concentration, standardisation is the process of determining the precise concentration of the solution. As precise concentration measurements are necessary for many scientific and industrial applications, it is a crucial stage in chemical analysis. In order to determine the concentration of the unknown solution, the standardisation procedure entails mixing a measured amount of the standard solution with a known quantity of the solution being tested. The primary standard, which is a pure material with a known and steady concentration, is often the standard solution. Standardization is used in many disciplines, including chemistry, biology, and engineering, to assure
Learn more about standardisation here:
https://brainly.com/question/30457109
#SPJ4
2.Use the figure to compare the melting points of the metals in Groups 1 and 2. As you go down the groups from top to bottom, what generally happens to the melting point?
3. As you go down a group in the periodic table, atomic radii generally increase. Based on the pattern you observed in Question 2, how is the melting point of a metal related to atomic radius?
4. Use the patterns you identified to estimate the likely melting point for K in group 1 and Ba in group 2. Give specific ranges in temperatures for each element and explain your reasoning.
5. Look at the melting points for the metals in the fourth and fifth periods of the periodic table in the figure. As you go from left to right in these periods, how does the melting point change?
6. Considering the patterns you have identified, estimate the likely melting points of Cd, V, and Co.

Answers
As we go down, it leads to weaker metallic bonding between the atoms. Weaker metallic bonding results in a lower melting point because it is easier to break the bonds between the atoms.
The melting point of a metal is inversely related to its atomic radius, i.e., as the atomic radius increases, the melting point decreases.
K belongs to Group 1 and Ba belongs to Group 2. As we go down these groups, the melting point decreases. Therefore, K will have a lower melting point than Na and Li, which are the elements above it in the group. The melting point of Na is about 370 K, and the melting point of Li is about 453 K. Therefore, the melting point of K is likely to be in the range of 336-370 K. Similarly, Ba will have a lower melting point than Ca and Mg, which are the elements above it in the group. The melting point of Ca is about 1115 K, and the melting point of Mg is about 923 K. Therefore, the melting point of Ba is likely to be in the range of 700-1115 K.
As we go from left to right in the fourth and fifth periods of the periodic table, the melting point generally increases. This is because the number of valence electrons increases, which leads to stronger metallic bonding and higher melting points.
Cd belongs to Group 12, V belongs to Group 5, and Co belongs to Group 9. As we go down Group 12, the melting point decreases, so Cd is likely to have a lower melting point than Zn, which is the element above it in the group. The melting point of Zn is about 693 K. Therefore, the melting point of Cd is likely to be in the range of 594-693 K. As we go from left to right in Group 5, the melting point generally increases. Therefore, V is likely to have a higher melting point than Ti, which is the element to its left. The melting point of Ti is about 1941 K. Therefore, the melting point of V is likely to be in the range of 1941-2183 K. As we go from left to right in Group 9, the melting point generally increases. Therefore, Co is likely to have a higher melting point than Ni, which is the element to its left. The melting point of Ni is about 1728 K. Therefore, the melting point of Co is likely to be in the range of 1728-1768 K.
To learn more about melting points please visit-
https://brainly.com/question/29578567
#SPJ1
In an experiment, the molar mass of the compound was determined to be 118. 084 g/mol. What is the molecular formula of the compound?
Answers
The molecular formula of the compound cannot be determined with certainty based on the given information alone. Further information is needed, such as the empirical formula or additional experimental data, to determine the molecular formula.
The molar mass of a compound is the sum of the atomic masses of all the atoms in one mole of the compound, and it can be used to calculate the molar mass ratio of the elements in the compound. However, the molar mass alone is not sufficient to determine the molecular formula of the compound.
For example, a compound with a molar mass of 118.084 g/mol could have a molecular formula of C9H10O, C4H14N2, C6H12O6, or many other possibilities. The determination of the molecular formula requires additional information about the relative numbers of atoms in the compound.
One possible approach to determine the molecular formula is by determining the empirical formula of the compound, which gives the simplest whole-number ratio of the elements in the compound. The empirical formula can be found experimentally by analyzing the mass percentages of the elements in the compound and using their atomic masses to calculate the empirical formula.
Once the empirical formula is known, the molecular formula can be determined based on the molar mass of the compound. For example, if the empirical formula is C3H5O and the molar mass is 118.084 g/mol, the molecular formula can be calculated as (C3H5O)n, where n is an integer that depends on the molar mass of the empirical formula relative to the molar mass of the compound.
The molecular formula of a compound cannot be determined with certainty based on the given information alone. Further information, such as the empirical formula or additional experimental data, is needed to determine the molecular formula. The determination of the empirical formula can be used to find the simplest whole-number ratio of the elements in the compound, which is a key step in determining the molecular formula.
To know more about molecular formula, visit:
https://brainly.com/question/15960587
#SPJ11
a sample of seawater has a mass of 158g and a volume of 156mL. What is its density?
Answers
A sample of seawater has a mass of 158g and a volume of 156mL. its density is 1.012 g/mL.
According to the question the data are given is as follows:
volume of sea water = 156 mL
mass of sea water = 158 g
density of sea water can be calculated by the following formula :
D = M / V
where,
D = density
M = mass of sample
V = volume of sample
substituting all the value in the given formula , we get :
D = 158 g / 156 mL = 1.012 g/mL
Density of seawater = 1.012 g / mL
Thus, A sample of seawater has a mass of 158g and a volume of 156mL. its density is 1.012 g/mL .
To learn more about Density here
https://brainly.com/question/15164682
#SPJ1
PLEASE HELP ASAP WILL GIVE BRAINLIEST
explain in terms of subatomic particles why the X+ ion is smaller than the X atom.
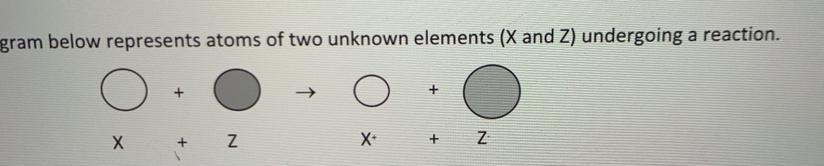
Answers
Answer:
X+ ion cation is smaller than neutral atom because when one or more electrons are lose by an atom it usually result in lose of shell.
And when electron is removed positive/ negative charged ratio increas which causes more effective nuclear charge.
The more effective nuclear charge the less its size.
Explanation:
In the equation below
1. If a sample containing 12.5 moles of NH3 is reacted with excess CuO, how many moles of each product can be made?
N2=
Cu=
H20=
thanks!

Answers
Answer:
6.25 moles of N₂ is produced, and 18.8 moles of Cu and H₂O is produced.
Explanation:
We are given the chemical equation:
\(\displaystyle 2\text{NH$_3$}_\text{(g)} + 3\text{CuO}_\text{(s)} \longrightarrow \text{N$_2$}_\text{(g)} + 3\text{Cu}_\text{(s)}+3\text{H$_2$O}_\text{(g)}\)
And we want to determine the amount of products produced when 12.5 moles of NH₃ is reacted with excess CuO.
Compute using stoichiometry. From the equation, we can see the following stoichiometric ratios:
The ratio between NH₃ and N₂ is 2:1. (i.e. One mole of N₂ is produced from every two moles of NH₃.)The ratio between NH₃ and Cu is 2:3. The ratio between NH₃ and H₂O is 2:3. (i.e. Three moles of H₂O or Cu is produced frome every two moles of NH₃.)Dimensional Analysis:
The amount of N₂ produced:\(\displaystyle 12.5\text{ mol NH$_3$} \cdot \frac{1\text{ mol N$_2$}}{2\text{ mol NH$_3$}} = 6.25\text{ mol N$_2$}\)
The amount of Cu produced:\(\displaystyle 12.5\text{ mol NH$_3$} \cdot \frac{3\text{ mol Cu}}{2\text{ mol NH$_3$}} = 18.8\text{ mol Cu}\)
And the amount of H₂O produced:\(\displaystyle 12.5\text{ mol NH$_3$} \cdot \frac{3\text{ mol H$_2$O}}{2\text{ mol NH$_3$}} = 18.8\text{ mol H$_2$O}\)
In conclusion, 6.25 moles of N₂ is produced, and 18.8 moles of Cu and H₂O is produced.
A main group metal was studied and found to exhibit the following properties: It does not occur free in nature. It loses valence electrons readily. It reacts with oxygen gas creating an oxide with a high melting point.
Answers
The metal in question is likely to be an alkali metal or an alkaline earth metal. These metals share many characteristics, including their high reactivity, the tendency to lose valence electrons and the formation of stable metal oxides.
Property A indicates that the metal is not found in its elemental form in nature, which is a characteristic shared by many reactive metals that readily combine with other elements. Alkali metals and alkaline earth metals are both highly reactive and do not occur in their elemental form in nature.
Property B suggests that the metal has a tendency to lose valence electrons, which is a characteristic of metals with low ionization energies. Alkali metals and alkaline earth metals have low ionization energies, and therefore, they are highly reactive and can easily lose their valence electrons to form cations.
Property C indicates that the metal reacts with oxygen to form an oxide with a high melting point. This property is consistent with the formation of metal oxides, which are typically formed when metals react with oxygen. The high melting point of the oxide suggests that it is a stable compound, which is consistent with the properties of alkali and alkaline earth metal oxides.
To learn more about alkali metals
https://brainly.com/question/18153051
#SPJ4
Complete question:
A main group metal was studied and found to exhibit the following properties:
A - It does not occur free in nature.
B - It loses valence electrons readily.
C - It reacts with oxygen gas creating an oxide with a high melting point.
One atom of element A bonds with element E.

Answers
Answer:
Ae4
Explanation:
which virtually showed the reaction between group 4 element and group 7 element
There are ________ mol of carbon atoms in 4 mol of C4H8O2
Answers
There are 16 mol of carbon atoms in 4 mol of C4H8O2
The chemical formula C4H8O2 tells us that each molecule of this compound contains 4 carbon atoms, 8 hydrogen atoms, and 2 oxygen atoms. Therefore, to determine the number of carbon atoms in 4 mol of C4H8O2, we need to multiply the number of moles by the number of carbon atoms per molecule:
Number of carbon atoms = number of moles × number of carbon atoms per molecule
Number of carbon atoms = 4 mol × 4 carbon atoms per molecule
Number of carbon atoms = 16 mol
So there are 16 mol of carbon atoms in 4 mol of C4H8O2. It's important to note that the chemical formula of a compound gives us information about the ratio of atoms in the compound, which allows us to determine the number of atoms in a given amount of the compound.
To know more about the Carbon, here
https://brainly.com/question/30282405
#SPJ4
I just need help finding the last part which is the % of composition of unknown Ni compound

Answers
The % of composition of unknown Ni compound is 5869 %
Composition is the act of combining part or element to form a whole and it is also refer to arrangement type and ratio of atom and molecule in the chemical substances
Here we have to find % composition of Ni compound = ?
For % composition = mass of element/molecular mass × 100
Mass of Ni = 58.69 u
For % composition of unknown Ni compound = 58.69 u × 100
For % composition of unknown Ni compound = 5869 %
% composition = 5869 %
Know more about Ni compound
https://brainly.com/question/6615107
#SPJ1
what type of wave is shown ?

Answers
how many molecules of oxygen are contain in 100 ml of air at NTP? (air contains 21% oxygen by volume
Answers
Answer:
600
Explanation:
because if there's 6 molecules in each then 6×100 is 600
HELP QUICKLY PLEASEEE

Answers
Answer:
Ionic bonds are when electrons are gained/lost. Hence the valence charges.
A FeCl 3 solution is 0.175 M. How many mL of a 0.175 M FeCl 3 solution are needed to make 650. mL of a solution that is 0.300 M in Cl - ion?
Answers
Based on the molar concentration, the volume of FeCl₃ required is 371 mL
What is the mole ratio of the reaction?The mole ratio of the reaction is determined from the equation of the reaction as follows:
Equation of reaction: FeCl₃ (aq) ----> Fe³⁺ (aq) + 3 Cl⁻ (aq)
From the equation of reaction, the mole ratio of FeCl₃ to Cl⁻ is 1 : 3
The moles of chloride ions in 650 mL of 0.300 M is calculated from the formula below:
Moles = molar concentration * volume in litersMoles of Cl⁻ ions = 0.300 * 650/100
Moles of Cl⁻ ions = 0.195
Moles of FeCl₃ required = 0.195/3
Moles of FeCl₃required = 0.065
volume of FeCl₃ required = 0.065 / 0.175
volume of FeCl₃ required = 0.371 L or 371 mL
Learn more about molar concentration at: https://brainly.com/question/14469428
#SPJ1
How many carbon atoms are in 1.25 mol of silver acetate?
Answers
The number of carbon atoms present in 1.25 mol of silver acetate is 1.875 × 10²³.
What is Avogadro's number?Avogadro's number is define as the number of atoms of any substance present in one mole of that substance, and it is equal to 6.022 × 10²³, i.e.
1 mole = 6.022 × 10²³ atoms/mol
Given moles of silver acetate = 1.25 moles
Number of atoms of silver acetate in 1.25 moles = 1.25 × 6.022 × 10²³ = 7.5×10²³
Molecular formula of silver acetate is CH₃CO₂Ag, means in this molecule total 8 atoms are present so number of carbon atom will be calculated as:
Carbon atoms = (2× 7.5 × 10²³) / 8 = 1.875 × 10²³ atoms [here we multiply by 2 because 2 atoms of carbon is present in olecule]
Hence required atoms of carbon is 1.875 × 10²³.
To know more about Avogadro's number, visit the below link:
https://brainly.com/question/1513182
What are indicators? How methyl orange and phenolphthalein changes their colour in acidic and basic solutions? How litmus paper changes its colour in different solutions?
Answers
Answer:
See the answer below
Explanation:
In chemistry, indicators are substances that are capable of changing colors with respect to the pH. Each indicator has its characteristic color in acidic pH and another characteristic color in alkaline pH.
Methyl orange indicator appears red in acidic solution and yellow in basic solutions. Phenolphthalein is usually colorless in acidic solutions and appears pink in basic solutions. A red litmus paper will turn blue in alkaline solutions while a blue litmus paper will turn red in acidic solutions.
When a single salt crystal is added to a cool liquid, large crystals form. Which statement about the original cool liquid is correct?
A) The liquid is a heterogeneous mixture because the crystals can be easily separated from the liquid
B) The liquid is a pure substance because it was made up of a single compound
C) The liquid is a pure substance because it was uniform before the crystal was added
D) The liquid is a homogenous mixture because the substance that formed the large crystals was uniformly dissolved
Answers
The liquid is a homogeneous mixture because the substance that formed the large crystals was uniformly dissolved.
A solution is composed of a solute and a solvent. A solution is a typical example of a homogeneous mixture.
We define a solution as any combination of solute and solvent. A solution can be regarded as a homogeneous mixture since the solute is uniformly distributed throughout the solution. The solute and solvent constitutes one phase.
When a salt crystal is added to the solution, crystallization is induced and large crystals of solute form.
https://brainly.com/question/9970247
A salt crystal is a cubic form of sodium chloride that is closely bounded together by the reaction of sodium and chlorine ions during electrovalent bonding.
Taking a look at a heterogeneous mixture;
It refers to the mixtures that are not thoroughly mixed and uniform in composition.
On the other hand, homogeneous mixtures are formed when a substance i.e. solute (here, it is the salt crystal) is being added to a cool liquid(solvent) is thoroughly mixed.
From the given information, we are being told that when this single salt crystal is added to the cool liquid, large crystals are formed.
This implies that the formation of large crystals is brought about by the action of crystaliization rendering the liquid to be a homogeneous mixture.
We can therefore conclude that the correct statement about the original cool liquid is in Option D.
Learn more about Solution here:
https://brainly.com/question/1616939?referrer=searchResults
When 20.0 grams of a substance is completely melted, 3,444 J are absorbed. What is the heat of fusion for this substance?
Answers
The heat of fusion for this substance is 172.2 J/gram. The heat of fusion represents the amount of heat energy required to change one gram of the substance from the solid phase to the liquid phase at its melting point.
To calculate the heat of fusion for a substance, we need to divide the amount of heat absorbed by the mass of the substance.
Given:
Mass of the substance (m) = 20.0 grams
Heat absorbed (Q) = 3,444 J
Heat of fusion (ΔHf) = Q / m
Plugging in the values:
ΔHf = 3,444 J / 20.0 g
Calculating the result:
ΔHf = 172.2 J/g
Therefore, the heat of fusion for this substance is 172.2 J/gram. The heat of fusion represents the amount of heat energy required to change one gram of the substance from the solid phase to the liquid phase at its melting point. It is a characteristic property of the substance and helps determine the amount of energy needed for phase transitions.
For more question on fusion
https://brainly.com/question/17870368
#SPJ11
What is the mass % of H in Mn(C₂H₂O₂)4?
Answers
The percent mass of H is 3.3%. This can be found from the data of the elements that we have in the problem.
How do you find the percent mass of an element in a compound?To find the percent mass of an element in a compound, you need to follow these steps:
Determine the molar mass of the compound: This is the sum of the atomic masses of all the elements in the compound. You can find the atomic masses of elements on the periodic table.
Determine the molar mass of the element: This is the atomic mass of the element as it appears in the compound.
Calculate the moles of the element in the compound: This can be done using the formula: Moles = Mass of element / Molar mass of element.
Calculate the percent mass of the element in the compound: This can be done using the formula: Percent mass = (Moles of element x Molar mass of element / Molar mass of compound) x 100%.
Molar mass of the compound = 239 g/mol
Mass of H present = 8 (1) = 8
Percent of H = 8/239* 100/1
= 3.3%
Learn more about percent mass:https://brainly.com/question/5394922
#SPJ1
3.
What is the oxidation number of oxygen (O) in O2?
0
+2
−2
+4
Answers
Liquid water can be separated into hydrogen gas and oxygen gas to electro sis. One mole of hydrogen gas in 0.5 moles of oxygen gas is produced from one mole of liquid water collected in a separate 10 L container at 1 atm. Will the temperatures of the gas be equal? In 1 to 2 sentences use the ideal gas law to explain your answer
Answers
Answer:
Explanation:
Liquid water can be separated into hydrogen gas and oxygen gas by
electrolysis. One mole of hydrogen gas in 0.5 moles of oxygen gas is produced from one mole of liquid water collected in a separate 10 L containers at 1 atm. Will the temperatures of the gas be equal? In 1 to 2 sentences use the ideal gas law to explain your answer
container A has 1 mole of H2, container B has 0.5 moles of O2
PV=nRT Each container has the SAME P and V , so container A must have a lower T since PV=1XRXT, while container B has 0.5XRXT and so T is greater in order to keep PV constant
,
The ideal gas law \(\text{(PV = nRT)}\) specifies the mechanical behavior of ideal gases. It is capable of calculating the volume of gases produced or consumed.
In chemical equations, these equations are widely used to convert between volumes and molar quantities.Use ideal gas law\(\to PV=n RT\\\\\)
Therefore
\(\text{V, R ,\ and\ P}\) is the same for both So, \(\bold{\frac{PV}{R}= nt }\) = same for both.When n increases, T decreases, so, n is 1 for \(H_2\) and 0.5 for \(O_2\), implying that \(H_2\) is hotter.Learn more:
brainly.com/question/3900938
en un recipiente de 5l se introduce un gas oxígeno de 4 atm ¿ que presion ejercera si duplicamos el volumen del recipiente sin que varíe sutemperatura?
Answers
Answer:
Density: if you take alcohol and look at its density and divide the alcohol into two containers the density will be the same.
Temperature: if you take an ice cream and split it, the temperature will be the same in the two pieces.
Explanation: