Answers
The average density of three trials are 3.61 g/mL
It is given that
Density in first trial d1 = 3.5512 g/mL
Density in second trial d2 = 3.4188 g/mL
Density in first trial d3 = 3.8617 g/mL
We have to find average density
Density is a measurement that compares the amount of matter an object has to its volume. An object with much matter in a certain volume has high density. An object with little matter in the same amount of volume has a low density.
Average density = sum of all density/number of density
Average density = d1 + d2 + d3
3
Average density =
3.5512 + 3.4188 + 3.8617
3
= 3.61 g/mL
Hence, average density of three trials are 3.61 g/mL
Learn more about Density here:
https://brainly.com/question/1354972
#SPJ1
Related Questions
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
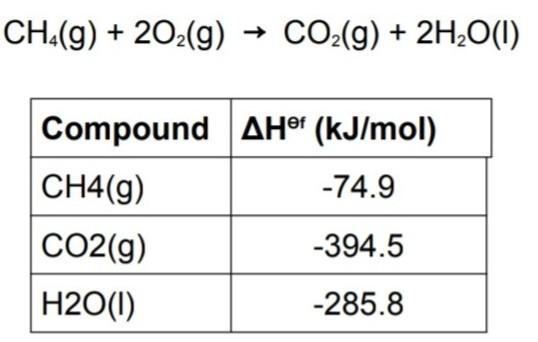
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
how much energy is required to vaporize 2 kg of copper
Answers
It would require approximately 600 kilojoules of energy to vaporize 2 kg of copper.
To calculate the energy required to vaporize a substance, we need to consider the heat of vaporization, which is the amount of energy required to convert a given amount of substance from its liquid state to its gaseous state at a constant temperature.
The heat of vaporization for copper is approximately 300 kJ/kg (kilojoules per kilogram) at its boiling point, which is around 2567 degrees Celsius (4649 degrees Fahrenheit). This means that for every kilogram of copper, 300 kJ of energy is needed to vaporize it.
Given that you have 2 kg of copper, we can calculate the total energy required as follows:
Energy = Heat of Vaporization × Mass
Energy = 300 kJ/kg × 2 kg
Energy = 600 kJ
Therefore, it would require approximately 600 kilojoules of energy to vaporize 2 kg of copper.
It's worth noting that the heat of vaporization can vary slightly depending on the purity of the copper and the specific conditions, such as temperature and pressure. The value provided here is an approximation. Additionally, it's important to handle copper and any high-temperature processes with caution, as they can pose safety hazards.
for more questions on vaporize
https://brainly.com/question/24258
#SPJ8
cyanate ion waste solution from gold-mining operations can be destroyed by treatment with hypochlorite ion in basic solution. Write a balanced oxidation-reduction equation for this reaction. OCN^-(aq) +OCl^-(aq) --> CO2^-(aq)+N2(g)+Cl^-(aq)+H2O(l)
Answers
The balanced oxidation-reduction equation for the destruction of cyanate ion waste solution from gold-mining operations by treatment with hypochlorite ion in basic solution is:
OCN⁻(aq) + OCl⁻(aq) + 2OH⁻(aq) → CO₂⁻(aq) + N₂(g) + Cl⁻(aq) + H₂O(l)
In this reaction, the cyanate ion (OCN⁻) is oxidized to carbon dioxide (CO₂⁻) and nitrogen gas (N₂), while the hypochlorite ion (OCl⁻) is reduced to chloride ion (Cl⁻). The reaction takes place in basic solution, which provides the hydroxide ions (OH⁻) needed to neutralize the acidic H⁺ ions produced during the oxidation of the cyanate ion.
The reaction is exothermic, releasing heat energy as the products form. This reaction is an effective way to dispose of the cyanate ion waste generated by gold-mining operations, as it converts the hazardous waste into harmless gases and ions.
To learn more about oxidation-reduction equation, here
https://brainly.com/question/13699873
#SPJ1
How is steel made from the raw product of the blast furnace known
as "pig iron"? What are the advantages of using steel?
List references used (if any were used) to answer this question.
Answers
Steel is produced from pig iron through a process known as steelmaking or iron and steel production.
The pig iron obtained from the blast furnace contains high amounts of carbon, impurities, and other elements. To convert pig iron into steel, the carbon content needs to be reduced to desired levels, and impurities must be removed.One common method of steelmaking is the basic oxygen process (BOP). In this process, pig iron is placed in a vessel called a converter, where oxygen is blown through the molten metal. The oxygen reacts with the carbon and impurities, causing them to oxidize and form gases that are released. Alloying elements and desired additives can be added at this stage to achieve specific steel properties. Another method is the electric arc furnace (EAF), where an electric arc is used to heat and melt the pig iron, allowing impurities to be oxidized and removed.The advantages of using steel are numerous. Steel is strong, durable, and versatile, making it suitable for a wide range of applications. It has high tensile strength, which means it can withstand heavy loads and pressures. Steel is also resistant to corrosion, making it ideal for construction, infrastructure, and transportation projects. It is a recyclable material, contributing to sustainability and reducing environmental impact. Additionally, steel can be fabricated into various shapes and sizes, allowing for customization and flexibility in design.References:
A. Ghosh and A. Chatterjee, Ironmaking and Steelmaking: Theory and Practice, PHI Learning, 2008.
R.H. Tupkary and V.R. Tupkary, An Introduction to Modern Iron Making, Khanna Publishers, 2010.
J.R. Davis, ed., ASM Specialty Handbook: Carbon and Alloy Steels, ASM International, 1995.
for such more questions on production
https://brainly.com/question/25597694
#SPJ8
be sure to include the correct physical states of reactants and products. write the balanced equation of the reaction that occurs in a voltaic cell made using aluminum and tin electrodes immersed in 1.00 m solutions of al3 and sn2 . use the standard electrode potentials listed below to answer this question.
Answers
the balanced equation of the reaction that occur in voltaic cell is 3sn2+ + 2AL(s) = 3sn(3) + 2AL3+.
Half of the cell reaction will occur at the cathode, while the other half will occur at the anode due to the Sanger value of the standard reduction potential. To generate an electric current, galvanic (or voltaic) cells use a thermodynamically favoured redox reaction. Each half-reaction occurs in its own compartment, or half-cell, which contains an electrode. The anode is indeed the electrolytic capacitor at which oxidation occurs, and the cathode is the electrode in which reduction happens. The galvanic cell, also recognised as the voltaic cell, is a kind of electrochemical cell decided to name after scientists Luigi Galvani and Alessandro Volta. Oxidation-reduction reactions Galvanic (or voltaic) cells use a thermodynamically favourable redox reaction to generate an electric current. Each half-reaction takes place in its own compartment, or half-cell, which is equipped with an electrode.
Learn more about voltaic cell here:
https://brainly.com/question/1370699
#SPJ4
state any two characteristics that prove water is a compound
Answers
Answer:
(a) Elements are combined in a specific ratio. 2 atoms of hydrogen and 1 atom of oxygen make water. (b) The properties of water are different from that of oxygen and hydrogen.
If solid ammonium fluoride (NH4F) is dissolved in pure water, will the solution be acidic, neutral, or basic?
Answers
Answer:
Dissolving NH4F in water will form a weak acidic solution.
Explanation:
That it is a weak acid solution means that it has a pH below 7 but close to the value, that is, it does not contain as many acids as those substances that are around a pH of 1 to 4, generally weak acids have a pH approximately 5 to 6
The solution of solid ammonium fluoride in pure water has been slightly acidic in nature.
Ammonium fluoride has been an ionic compound formed by the interaction of cationic ammonia and anionic fluoride ions. The dissolution of ionic compounds will result in the compound in its dissociated ionic state.
The dissociation results in the formation of ammonium cation. The ammonium has been a strong acid.
The resulted anion has been fluoride. It has been a strong base, but slightly weaker than ammonia.
Thus the resultant solution will result in slightly acidic nature.
For more information about ammonium fluoride, refer to the link:
https://brainly.com/question/20180239
5 points
13. Silver (Ag) has 47 protons in each atom. Based on this
information, which of the following also describes an atom of
silver?
0 It has no neutrons.
It has 47 electrons.
O It has 23 neutrons and 24 electrons.
It has a total of 94 neutrons and electrons.
Answers
Answer:
C
Explanation:
The number of protons have to be the same of that of electrons or silver will be an ion.
It is not possible to have no neutrons since the mass of electrons are negligible.
Find the number of grams in 4.26 X 1024 formula units of MgCl2.
Answers
Answer:
Explanation:
Number of moles of Cl atoms in 1.20 1024 Formula units of magnesium chloride mgcl2
The number of moles of Cl is twice as much, because the ratio of Cl in MgCl2 to MgCl2 is 2:1. Therefore, there are 12 moles of Cl. There are 7.22×1024 atoms Cl in 3.61×1024 formula units of MgCl2
The number of grams in 4.26 × 10²⁴ formula units of MgCl₂ is equal to 673.06 grams.
What is the relation between moles and mass?Mass of any substance from moles will be calculated by using the below equation as:
n = W/M, where
W = given mass
M = molar mass
And relation between moles and atoms per moles is:
In 1 moles = 6.022 × 10²³ atoms are present
Given number of atoms of MgCl₂ = 4.26 × 10²⁴
Moles of MgCl₂ = 4.26 × 10²⁴atoms / 6.022 × 10²³ atoms/mol = 7.07 mol
Non we convert this moles of MgCl₂ into grams by using the above given formula as:
W = (7.07mol)(95.2g/mol) = 673.06 g
Hence required mass of MgCl₂ is 673.06g.
To know more about moles & mass, visit the below link:
https://brainly.com/question/15373263
state four temperature scales
Answers
Answer: Celsius, Fahrenheit, Kelvin, and Rankine.
Hope this helped you!
You have three crystal substances (x, y, and z) whose properties are listed in the table below. Identify the types of bonds in each substance and explain your answer.
Answers
X contains metallic bond
Y contains ionic bond
Z contains covalent bond
What is the crystal structure?We know that the crystal structure of a compound would have to do with the way that the atoms and the elements that compose the compound could be said to have been arranged. Thus if a compound is said to have a crystal structure then we can say that the compound would be crystalline in nature as we can see from the table that we have in the question here.
The kind of bonds that we have in the compound is about one of things that can be able to determine whether or not the compound has a crystalline structure as we can see.
Looking at the properties of the compounds that have been shown in the bale that has been attached to the answer that we have here, we can be able to make a decision regarding each of the substances shown.
Learn more about crystal substances:https://brainly.com/question/28728101
#SPJ1

which gas is fossil fuel
Answers
Answer:
methane
Explanation: methane is obtained from the decaying of flora and fauna mostlyunder damp
Which statement(s) about neutrons is/are TRUE?
SELECT ALL THAT APPLY
The major clue to their existence was extra mass in
a atoms that protons and electrons could not
account for.
b They were observed and measured directly, like
protons and electrons were.
C They have the same mass as protons.
d They have a negative charge.
Answers
Answer:
C
Explanation:
Because D ask "They have a negative charge" yes and no, but neutrons do, Why? Well because the neutron has a negative charge both in its inner core and its outer edge, with a positive charge sandwiched in between to make the particle electrically neutral. Whilst B is wrong because like protons and electrons they were both measured and observed differently, just as the neutron. So that makes C the only correct answer.
You dissolve 0.26 moles of Co(NO;) in 0.30L of water. The resulting concentration is 0.87.M.For an experiment, you need a concentration of 0.30.M. What volume of water is needed for thisconcentration to result?
Answers
According to the explanation given in a previous session, now we have the same type of question but in a different situation, we still can use the same formula and change what we are looking for now, let's set up the Dilution formula again:
M1V1 = M2V2
We have:
M1 = 0.87 M
V1 = 0.30 L
M2 = 0.30 M
V2 = ?
Now we add the values into the formula:
0.87 * 0.30 = 0.30 * V2
0.261 = 0.30V2
V2 = 0.87 L, this will be the new volume
How many liters of chlorine are needed to react with 31.2
Answers
Answer:
Explanation: all the answer for the question is that link
Write a lab report for this lesson’s lab. Be sure that your report: includes all major elements of a lab report. meets your teacher’s content and format expectations. is clearly organized and formatted. demonstrates strong scientific reasoning and writing.
Answers
Lab Document: Examining How Temperature Affects Enzyme Activity Introduction:
Title: The Impacts of Temperature on Compound Action.
Question: How does temperature affect catalase activity?
Variables: Temperature is the independent variable, and enzyme activity rate is the dependent variable.
Materials and Method:3% solution of hydrogen peroxide Potato slices Test tubes Thermometer Ice bath Water bath Hot water bath
Slice the potato into equal-sized pieces.
Label three test tubes with the labels "Room temperature," "Ice bath," and "Hot water bath."
Place the test tube marked "Ice shower" in a receptacle loaded up with ice water for 5 minutes.
For five minutes, place the "Room temperature" test tube on the lab bench.
Each test tube should contain one potato slice.
Measure the time it takes for the potato slice to sink to the bottom of each test tube and observe the reaction.
Control and Experimental Groups:The three test tubes containing potato slices and a solution of hydrogen peroxide make up the experimental group. The same setup is used in the control group, but there are no potato slices.
Results:At low temperatures, the reaction rate was sluggish, and at high temperatures, the enzyme was denatured.
Question incomplete:Write a lab report for this lesson’s lab. Be sure that your report: includes all major elements of a lab report. meets your teacher’s content and format expectations is clearly organized and formatted. demonstrates strong scientific reasoning and writing.
While writing, you can revisit previous parts of the lesson by returning to the course map. Be sure to refer to the
lab’s student guide, which you can find on the first page of the lab experiment activity. You may also find it
helpful to refer to the remaining pages of this guide, which provide general guidelines for writing lab reports.
Lab Report Checklist
Introduction
Did you title your lab report?
Did you state the purpose of the experiment?
Did you state the question you posed before the
experiment?
Did you restate the hypothesis (or prediction) you
formulated before the experiment?
Did you list all variables and label the
independent and dependent variables? Did you
indicate any controlled variables?
Materials and Procedure
Did you make a list of materials? Did you include
quantities and SI units?
Did you present the steps of the procedure as a
numbered list? Did you note any changes to the
original procedure?
Did you identify your experimental and control
groups?
Learn more about lab report:
brainly.com/question/29500102
#SPJ1
10 cm3 of metallic cylinder has a mass of 39.35 g. The density of this cylinder is
Group of answer choices
3.935 g/cm3
39.35 g/cm3
10 g/cm3
5.935 g/cm3
Answers
Answer:3.935g/cm3
Explanation:39.35 g. /10cm3 = 3.935g/cm3
10 cm³ of metallic cylinder has a mass of 39.35 g. The density of this cylinder is 3.935 g/cm³.
Density is a measure of the amount of mass per unit volume of a substance. It is an intensive physical property, meaning that its value does not change depending on the size of the object.
Density is calculated by dividing an object’s mass by its volume.
The density of water is 1,000 kg/m³ at 4°C. The unit of density is kg/m³ (kilogram per cubic meter). The SI unit of density is kg/m³
Density = mass/volume
= 39.35 g/10 cm³
=3.935 g/cm³.
To know more about density here
https://brainly.com/question/29775886
#SPJ2
Calculate the solubility of nitrogen (in M) when the gas is at a pressure of
a) 2.00 atm
b) 688 mmHg
show steps please!
Answers
A.) The solubility of nitrogen at a pressure of 2.00 atm is \(1.36 \times 10^{(-3)} M.\)
B.) The solubility of nitrogen at a pressure of 688 mmHg is \(6.17 \times 10^{(-4)} M.\)
To calculate the solubility of nitrogen (N2) in M (molarity) at different pressures, we need to use Henry's Law, which states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. The equation for Henry's Law is:
C = k * P
Where:
C is the solubility of the gas in M (molarity)
k is the Henry's Law constant
P is the partial pressure of the gas
For nitrogen, the Henry's Law constant (k) is approximately 6.8 x 10^(-4) M/atm.
a) To calculate the solubility of nitrogen at a pressure of 2.00 atm:
C = (6.8 x 10^(-4) M/atm) * (2.00 atm)
C = 1.36 x 10^(-3) M
Therefore, the solubility of nitrogen at a pressure of 2.00 atm is 1.36 x 10^(-3) M.
b) To calculate the solubility of nitrogen at a pressure of 688 mmHg:
First, we need to convert mmHg to atm by dividing by 760 (since 1 atm = 760 mmHg).
P = 688 mmHg / 760 mmHg/atm
P = 0.905 atm
C = (6.8 x 10^(-4) M/atm) * (0.905 atm)
C = 6.17 x 10^(-4) M
Therefore, the solubility of nitrogen at a pressure of 688 mmHg is 6.17 x 10^(-4) M.
It's important to note that the solubility of a gas can also depend on temperature, so these calculations assume a constant temperature. Additionally, Henry's Law is an approximation and may not hold true for all gas-liquid systems, especially at high pressures or when there are significant intermolecular interactions between the gas and liquid.
For more question on pressure visit:
https://brainly.com/question/24719118
#SPJ8
A common carboxylic acid used in cooking is acetic acid, if its composed of two carbons whats its iupac name
Answers
Answer:
ethane
Explanation:
Ethane is an organic chemical compound with chemical formula C ₂H ₆. At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining
What is the frequency of
an electron with a wavelength
of 225 nm?
Answers
Answer:
1.33 x 10¹⁵ Hz
Explanation:
You can find the frequency using the following equation:
f = c / λ
In this equation,
-----> f = frequency (Hz)
-----> c = speed of light (3.0 x 10⁸ m/s)
-----> λ = wavelength (m)
Before we can plug the values into the equation, we need to convert the wavelength from nm to m.
225 nm 1 m
---------------- x -------------------- = 2.25 x 10⁻⁷ m
1 x 10⁹ nm
f = c / λ
f = (3.0 x 10⁸ m/s) / (2.25 x 10⁻⁷ m)
f = 1.33 x 10¹⁵ Hz
The frequency of a wave can be calculated using the wavelength and and speed of light. The frequency of a wave with 225 nm is 1.33 × 10¹⁵ Hz.
What is frequency ?Frequency of a wave is the number of wave cycles per second. It mathematically taken as the inverse of time taken to travel and it have a unit of s⁻¹ other than Hz.
The wavelength of an electromagnetic wave is the distance between two consecutive crests or troughs.Wavelength and frequency are in inverse relationship. For a longer wavelength the frequency will be lower.
Higher frequency waves are more energetic than longer wavelengths. Thus energy and frequency are in direct proportion
It is given that the wavelength of the electron is 225 nm. Consider the electron behaving as wave and the frequency v can be calculated using the speed of light c as follows:
\(v = C/\lambda\)
= (3 ×10⁸ m/s) /(225 ×⁻⁹ m)
= 1.33 × 10¹⁵ Hz.
Hence, the frequency of an electron with a wavelength of 225 nm is 1.33 × 10¹⁵ Hz.
To find more about frequency, refer the link below:
https://brainly.com/question/14316711
#SPJ2
9. Which atom is more likely to make a positive charge?
Oxygen or Hydrogen
Answers
Which molecule contains a nonpolar covalent bond?
A. HCI
B. F2
C. CO2
D. NH3
Answers
Mark if correct!
Please answer !! For chemistry

Answers
Answer:
a. is the correct answer
Explanation:
Could someone draw me electron dot diagrams? The Hydride Ion, Hydrogen Ion, Aluminum Ion, Nitride Ion, Oxide Ion, and Calcium Ion, and Sodium-Ion dot structures? Thanks!
Answers
Answer:
urorrr
Explanation:
hahhahahahahhaahah oki nam omy mymy mymy
Assuming that the mass defect originates solely from the interactions of protons and neutrons in the nucleu, estimate the nuclear binding energy of Li given yhe following data.observee atomic mass (a.m.u) of Li is 7.01600u
1u=1.66054×10^-27kg
Electron rest mass= 9.10939×10^-31kg
Proton rest mass=1.67262×10^-27kg
Neutron rest mass=1.67493×10^-27kg
C=2.998×10^8
Answers
The mass lost in the formation of a nucleus is converted into energy in accordance with the Einstein's mass energy relationship and released, thereby tending to impart stability to the nucleus.
An input of the same amount of energy referred to as the binding energy would be required to decompose the nucleus into its component nucleons. So the energy released from the nucleons during the formation of a nucleus is called the binding energy.
No: of protons in 'Li' = 3
No: of neutrons in 'Li' = 4
Total mass of nucleons = 3(1.67262×10⁻²⁷) + 4 (1.67493×10⁻²⁷) = 1.171 × 10⁻²⁶ kg
Mass defect = 1.171 × 10⁻²⁶ - 1.165 × 10⁻⁻²⁶ = 6 × 10⁻²⁹
Binding energy = Δmc² = 6 × 10⁻²⁹ × (2.998×10⁸)² = 5.392 × 10⁻¹² J
To know more about Binding energy, visit;
https://brainly.com/question/30087556
#SPJ1
PLS HELP WILL GIVE BRAINLYEST What happens when the magnet is removed from the coil in a circuit?
a.
The current stops
c.
The voltage decreases
b.
The current changes direction
d.
Power output increases
Answers
Answer:
b
Explanation:
Changing from solid to a liquid at or above melting point
is
Answers
Answer:
liquefaction
liquefaction is a process when a something turns into a liquid.
Explanation:
The best thermometer to use where temperatures drop below -39°C (-38.2°F) is:_______.
Answers
Answer:
The best thermometer to use for this temperature is the alcohol-in-a -glass thermometer.
Explanation:
The alcohol thermometer is a type of thermometer which uses ethanol as its thermometric liquid. This has some advantages at low temperatures because ethanol freezes at about −114.9 °C that is (−174.82 °F).
Due to its low freezing point, it can still function effectively as a thermometric liquid, expanding and contracting properly based on the applied heat, when dealing with temperature measurements that are at about -38.2°F.
The ionic compound MX(s) is formed from the metal M(s) and the diatomic gas X2(g) at standard conditions. Calculate the lattice energy given the following data( data in picture)

Answers
The lattice energy of MX is 459.2 kJ/mol.
The lattice energy (ΔH° lattice) of an ionic compound is the energy released when one mole of the solid is formed from its constituent gaseous ions under standard conditions. The lattice energy is calculated using the Born-Haber cycle, which involves several steps including atomization, ionization, dissociation, and sublimation energies.
The lattice energy is related to the Coulombic attraction between the oppositely charged ions in the solid. To calculate the lattice energy for MX, we can use the following equation:
ΔH° lattice = ΔH° sub + ΔH° ion + ΔH° diss + ΔH° formation
where ΔH° sub is the sublimation energy of M(s), ΔH° ion is the first ionization energy of M(g), ΔH° diss is the dissociation energy of X2(g), and ΔH° formation is the enthalpy of formation of MX(s).
Using the given data, we can calculate each of these values and substitute them into the equation to obtain the lattice energy. The final answer should be in units of kJ/mol.
ΔH° sub (M) = 107.3 kJ/mol
ΔH° ion (M) = 577.5 kJ/mol
ΔH° diss (X2) = 242 kJ/mol
ΔH° formation (MX) = -467.6 kJ/mol
ΔH° lattice = 107.3 + 577.5 + 242 + (-467.6) = 459.2 kJ/mol
As a result, MX has a lattice energy of 459.2 kJ/mol.
To know more about the Ionic compound, here
https://brainly.com/question/1603676
#SPJ1
Alls - -0.g) -
AL), |
When this equation is correctly balanced using
smallest whole numbers, what is the coefficient of O
2(g)?
A) 6
B) 2
C) 3
D) 4