Answers
Considering the definition of pH, the pH of a 3.4×10⁻⁶ M solution of HNO₃ is 5.47.
Definition of pHpH is a measure of acidity or alkalinity and indicates the amount of hydrogen ions present in a solution or substance.
The pH is defined as the negative base 10 logarithm of the activity of hydrogen ions:
pH= - log [H⁺]
pH in this caseStrong acids are those that are completely, or almost completely, dissociated in dilute solution (of concentration less than 0.1 M). So the concentration of protons is equal to the initial concentration of acid.
HNO₃ is a strong acid. So [H⁺]= [HNO₃]= 3.4×10⁻⁶ M
So, the pH can be calculated as:
pH= - log (3.4×10⁻⁶ M)
Solving:
pH= 5.47
Finally, the pH is 5.47.
Learn more about pH:
brainly.com/question/3992824
#SPJ1
Related Questions
I really need help please?

Answers
The petrol additive tetraethyl-lead (IV), Pb(C2H3)4, is now
banned in many countries. When it is completely burned in air,
lead (II) oxide, CO, and H2O are formed. How many moles of
oxygen are required to burn one mole of Pb(C2H5)?
Answers
Answer:
9.5 mol
Explanation:
Let's consider the unbalanced equation for the combustion of tetraethyl-lead (IV).
Pb(C₂H₅)₄ + O₂ ⇒ PbO + CO + H₂O
Pb atoms are balanced. We will balance C atoms by multiplying CO by 8.
Pb(C₂H₅)₄ + O₂ ⇒ PbO + 8 CO + H₂O
Then, we will balance H atoms by multiplying H₂O by 10.
Pb(C₂H₅)₄ + O₂ ⇒ PbO + 8 CO + 10 H₂O
Finally, we get the balanced equation by multiplying O₂ by 9.5.
Pb(C₂H₅)₄ + 9.5 O₂ ⇒ PbO + 8 CO + 10 H₂O
All in all, we need 9.5 moles of O₂ to burn 1 mole of Pb(C₂H₅)₄.
Describe the trend of the reactivity of the elements in group VII
Answers
The non-metal elements in Group 7 – known as the halogens – get less reactive as you go down the group
Answer & Explanation:
The reactivity of elements in Group VII, also known as Group 17, decreases with increasing atomic radius. This is because halogens have high electronegativities and a proclivity to gain electrons in noble gas configurations. Myths are traditional stories or beliefs that explain cultural or societal beliefs, customs, or natural phenomena. They can be passed down through generations and can be based on true or fictitious events. Mythology, on the other hand, is the collection of myths associated with a specific culture or religion. Mythology can be amplified through retelling, incorporation into religious practices; association with significant events or figures, and adaptation into other media forms such as literature, film, or art.
What does +430 degrees celsius feel like?
Answers
At this temperature, objects would be glowing red or even white-hot. It is important to note that human skin would suffer immediate and severe burns upon contact with surfaces at such temperatures. Additionally, the air would be scorching and potentially unbreathable due to the heat.
In summary, +430 degrees Celsius would be an intensely hot and dangerous environment, far beyond what humans can tolerate or safely experience.
- I Hope This Helps! :)
• Please Give Brainliest
80 POINTS
Someone pls help me out

Answers
2) The heat capacity of aluminum is 219.44 J/mol.°C.
3) a) the experimental ΔHs of ice is -0.154 kJ/mol.
b) too high
How to calculate heat capacity?Calculate the heat released by the aluminum:
q = mcΔT
where q = heat released, m = mass of aluminum, c = specific heat capacity of water and ΔT = change in temperature.
q = (24.7 g) (0.903 J/g°C) (100.0°C - 23.4°C)
q = 18643.26 J
Next, calculate the heat absorbed by the calorimeter:
q = mcΔT
q = (99.5 g + 24.7 g) (15.8 J/°C) (23.4°C - 19.5°C)
q = 4009.92 J
The heat released by the aluminum is equal to the heat absorbed by the calorimeter and water:
18643.26 J = 4009.92 J + q3
where q3 = heat absorbed by the water.
q3 = 14633.34 J
Calculate the molar heat capacity of aluminum:
Cp,m = q3 / (nΔT)
where Cp,m = molar heat capacity, n = number of moles of aluminum, and ΔT = change in temperature.
n = m / M
where m = mass of aluminum and M = molar mass of aluminum (26.98 g/mol).
n = 24.7 g / 26.98 g/mol
n = 0.916 mol
Cp,m = 14633.34 J / (0.916 mol * 76.6°C)
Cp,m = 219.44 J/mol.°C
Therefore, the heat capacity of aluminum is 219.44 J/mol.°C.
3) (a) To calculate the experimental ΔHs of ice, we first need to calculate the heat gained by the water and the heat lost by the ice during the process.
Heat gained by water = mass of water × specific heat capacity of water × change in temperature
= 100.0 g × 4.184 J/g·°C × (-20.1°C)
= -8,423.84 J
Heat lost by ice = mass of ice × heat of fusion of ice
= 25.6 g × 6.01 kJ/mol
= 154.496 J
Since the process is assumed to be adiabatic (no heat exchange with the surroundings), the heat gained by the water must be equal to the heat lost by the ice.
Thus, -8,423.84 J = 154.496 J = -8,269.344 J
The negative sign indicates that the process is exothermic. Therefore, the experimental ΔHs of ice is:
ΔHs = -154.496 J/mol = -0.154 kJ/mol
(b) If the student forgets to include the calorimeter term in the calculation, the calculated ΔHs of ice will be too high. This is because the heat absorbed by the calorimeter during the process is not accounted for, leading to an overestimation of the heat gained by the water and underestimation of the heat lost by the ice.
Find out more on heat capacity here: https://brainly.com/question/16559442
#SPJ1
5.712 grams = kilograms
Answers
Answer:
0.005712
Explanation:
5.712 grams = 0.005712 kilograms
Answer:
It would be 0.005712
Explanation:
PLEASE HELP!
Distilled vinegar contains a solution of acetic acid (CH3CO2H) in H2O. Using the formula M1V1=M2V2, solve for the concentration of the solution that results from diluting 0.50 L of 0.839 M vinegar solution to 2.5 L?
Question 4 options:
0.15 M
0.24 M
0.17 M
1.49 M
Answers
M1V1 = M2V2
0.839 M x 0.50 L = M2 x 2.5 L
M2 = (0.839 M x 0.50 L) / 2.5 L
M2 = 0.168 M
Therefore, the concentration of the solution that results from diluting 0.50 L of 0.839 M vinegar solution to 2.5 L is 0.168 M.
Answer: 0.17 M (rounded to two significant figures)
According to the diagram most stars are classified as?
O White Dwarfs
O Main Sequence
O Giants

Answers
Which of the ions Pd2+ , Ru2+ , Rh3+, and Hg2+ has an electron configuration of nd6 (n=3,4,5,…) ?
Answers
The ion with an electron configuration of nd6 is Pd²+.
The electron configuration of an atom or ion is the dispersion of electrons in its orbitals. It describes how electrons are grouped in an atom's or ion's many energy levels and sublevels.
The number of electrons in each energy level and sublevel is often shown in shorthand notation in the electron configuration. Carbon's electron configuration, for example, can be represented as:
1s² 2s² 2p²
This means that carbon has a total of 6 electrons, with 2 in the 1s orbital, 2 in the 2s orbital, and 2 in the 2p orbital.
An ion's electron configuration may differ from that of its parent atom due to electron loss or gain.
learn more about electron configuration here
https://brainly.com/question/26084288
#SPJ1
how solid particles are arranged
Answers
Explanation:
The particles in solids are arranged in a regular way. The particles in solids move only by vibrating about a fixed position. This gives solids a fixed shape and means that they cannot flow like liquids. The hotter a solid gets, the faster its particles vibrate.
Fatty foods become rancid due to the process of
Answers
Answer:
Fatty foods become rancid due to the process of rancidity
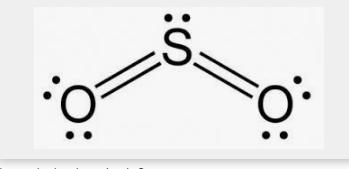
can you draw so2 lewis strucure i sent a picture what i did and u can explain if its wrong

Answers
Answer
The Lewis structure of SO2 (stable form) is shown below;
The unstable form SO2 Lewis structure is also shown below:

Can i have the answers

Answers
Answer:
Explanation:
NO₂ --> NO + O₂
We need the same number of each atom on both sides of the equation.
There is 1 nitrogen on the left and right sides of the equation. At this point, the nitrogens are balanced on both sides of the equation.
There are 2 oxygens on the left side and a total of 3 oxygens on the right side (1 oxygen in NO and 2 oxygens in O₂). The oxygens are not balanced.
We can place a 2 in front of NO₂ on the left side and 2 in front of NO on the right side. This will give 4 oxygens on the left side and a total of 4 oxygens on the right side.
We also have now 2 nitrogens on the left side and 2 on the right side.
2 NO₂ --> 2 NO + O₂
The equation is balanced with 2 nitrogens and 4 oxygens on both sides of the equation.
Calculate the pH when 64.0 mL of 0.150 M KOH is mixed with 20.0 mL of 0.300 M HBrO (Ka = 2.5 × 10⁻⁹)
Answers
Answer:
The answer is "12.06"
Explanation:
Given:
\(M(HBrO) = 0.3\ M\\\\V(HBrO) = 20 \ mL\\\\M(KOH) = 0.15 \ M\\\\V(KOH) = 64 \ mL\)
\(\to mol(HBrO) = M(HBrO) \times V(HBrO) = 0.3 M \times 20 mL = 6 \ mmol\\\\\to mol(KOH) = M(KOH) \times V(KOH)= 0.15 M \times 64 mL = 9.6 mmol\)
6 mmol of both will react
excess KOH remaining\(= 3.15 \ mmol\)
Volume\(= 20 + 64 = 84 \ mL\)
\([OH^{-}] = \frac{ 9.6 \ mmol}{84\ mL} = 0.01142\ M\)
use:
\(pOH = -\log [OH^-]\)
\(= -\log (1.142\times 10^{-2})\\\\= 1.94\)
use:
\(PH = 14 - pOH\)
\(= 14 - 1.94\\\\= 12.06\)
The pH of the resulting solution is 12.63.
The equation of the reaction is;
HBrO(aq) + KOH(aq) ------> KOBr(aq) + H2O(l)
Number of moles of KOH= 64/1000 × 0.150 M = 0.0096 moles
Number of moles of HBrO = 20/1000 × 0.300 M = 0.0060 moles
Number of moles of excess base = 0.0096 moles - 0.0060 moles = 0.0036 moles
Total volume of solution = 64.0 mL + 20.0 mL = 84 mL = 0.084 L
Molarity of excess base = 0.0036 moles/0.084 L = 0.043 M
pOH = -log[OH-]
pOH = -log[ 0.043 M]
pOH = 1.37
pH + pOH = 14
pH = 14 -pOH
pH = 14 - 1.37
pH = 12.63
Learn more: https://brainly.com/question/11897796
For the following statements about chemical equations, check the boxes that represent true statements.

Answers
1) Which statements are true?
Statement 1: TRUE.
The coefficients in a balanced chemical equation should be the smallest integers possible.
Statement 2: FASLE.
A chemical reaction is written like this:
\(Reactants\rightarrow Products\)Statement 3: TRUE.
We use the formulas and the states of the compounds in a chemical reaction.
Statement 4: FALSE.
We cannot change the identity of any compound involved in a chemical reaction. That's why we cannot change the subscripts.
Statement 5: FALSE.
The notation (aq) means aqueous. It means that the compound is dissolved in water.
1 and 3 are TRUE statements.
Part 2 The student wanted to know if the value obtained from their experiment (part 1) is similar to that calculated using average bond enthalpy data.
a) Using the balanced equation and the data in the table below, calculate the theoretical enthalpy of combustion.
Note: you will need to include the enthalpy of vaporisation for the liquid components which are also given.
C₂H5OH()+302(g) → 2CO2(g) + 3H₂O(1)
Average Bond Enthalpies (kJ mol-¹)
C-H 412
C-C 348
C-O 358
O=O 496
C=O 743
O-H 463
Enthalpy of Vaporisation (kJ mol-¹)
Ethanol 42.5
Water 41
Answers
-1113.5kJ is the theoretical enthalpy of combustion.
What makes energy different from enthalpy?
The entire amount of heat energy that is either absorbed or released in a thermodynamic system is measured by enthalpy. Internal energy denotes all of the potential or moving energy present in a thermodynamic system.
Enthalpy of combustion is the term used to describe the change in a system's enthalpy that occurs when one mole of a substance fully burns in oxygen or air at a specific temperature.
C₂H5OH()+302(g) → 2CO2(g) + 3H₂O(1)
Reactants:
5 C-H : 5*412
1 C-C : 348
1 C-O: 358
3 O=O: 3* 496
1 O-H: 463
Products:
2*2 C=O : 4*743
2*3 O-H: 6*463
Enthalpy of Vaporization (kJ mol-¹) for :
Ethanol 42.5
Water 41
Enthalpy of combustion : (5*412 + 348 + 358 + 3* 496 + 463 + 42.5) - ( 3*41 + 4*743 + 6*463)
: -1113.5kJ
To learn more about enthalpy use :
https://brainly.com/question/5374936
#SPJ1
What does it take in order for plates to move?
Answers
Answer:
Earth's thin outer shell is broken into big pieces called tectonic plates. These plates fit together like a puzzle, but they're not stuck in one place. They are floating on Earth's mantle, a really thick layer of hot flowing rock. The flow of the mantle causes tectonic plates to move in different directions.
Explanation:
Write the equation for the equilibrium constant (K) of the reaction studied in this exercise.
2C04 2- (ag) + 2Ht (ag) = CI20, 2- (ag) + H20(1)
Answers
The equation for the equilibrium constant (K) of the reaction studied in this exercise can be written as follows: K = ([\(CI_20\), 2-] * [\(H_20\)(1)]) / ([\(C0_4^ 2\)-] * [Ht])
In this equation, the concentrations of the species involved in the reaction are represented by the square brackets [ ]. The subscripts indicate the stoichiometric coefficients of each species in the balanced chemical equation.
The reaction being studied involves the following species:
\(C0_4^ 2\)- (ag) + 2Ht (ag) = \(CI_20\), 2- (ag) + \(H_20\)(1)
In the equilibrium constant expression, the concentration of \(CI_20\), 2- is multiplied by the concentration of \(H_20\)(1) and divided by the product of the concentrations of \(C0_4^ 2\)- and Ht. The stoichiometric coefficients in the balanced equation are used as exponents for the concentrations of the respective species.
It is important to note that the concentrations used in the equilibrium constant expression should be in molar units (mol/L) or expressed as partial pressures for gases.
Additionally, the equilibrium constant is specific to a given temperature, and its value provides information about the relative amounts of reactants and products at equilibrium.
For more such question on equilibrium constant visit:
https://brainly.com/question/3159758
#SPJ8
Need help on 2 and 6,7,8,9,10,11,12,13 please. If you know them.

Answers
Answer:
2. Sunlight + Water + Carbon dioxide + Chlorophyll → Glucose + Oxygen
6. A. Oxygen
7. C. Cellular respiration
8. A. Chemical energy
9. C. Lactic acid
10. B. Chlorophyll
11. C. Oxygen
12. D. Sunlight
13. A. Metabolism
Explanation:
I have been able to answer questions 2,6,7,8,9,10,11,12,13.
The mitochondria is the site where cellular respiration is carried out. During fermentation, energy is released and such process does not require oxygen. Lactic acid is built up when the muscles overwork themselves. The acid ferments when there is lack of oxygen and when that lack is greater than the energy used.
The process of photosynthesis results in the production of glucose and the release of oxygen which man takes in. Chlorophyll traps sunlight and turns it into chemical energy. But it's not actually all the light energy that are absorbed.
A platinum solid weighs 175 g. Look up its density and calculate the volume in dm3 this platinum solid occupies.
Answers
The volume of the platinum solid is determined as 8.16 cm³.
Volume of platinum solid
The volume of the platinum solid is calculated from its density and mass as shown below.
V = m/ρ
where;
V is the volume of the platinum solidm is the mass of the platinum solidρ is the density of the platinum solid = 21.45 g/cm³Volume of the platinum solid is calculated as follows;
V = 175 g / 21.45 g/cm³
V = 8.16 cm³
Thus, the volume of the platinum solid is determined as 8.16 cm³.
Learn more about platinum solid here: https://brainly.com/question/13261338
#SPJ1
I WILL GIVE BRAINLYEST
Au(NO3)3 + KCl --->
Answers
Isopentyl acetate (C7H14O2) is the compound responsible for the scent of bananas. A
molecular model of isopentyl acetate is shown in the margin below. Interestingly, bees
release about 1 mg (1 3 1026
g) of this compound when they sting. The resulting scent
attracts other bees to join the attack. How many molecules of isopentyl acetate are
released in a typical bee sting? How many atoms of carbon are present
Answers
The number of molecules in 1 g of isopentyl acetate, C₇H₁₄O₂ is 4.21 * 10²¹ molecules.
What is the number of molecules in one gram of isopentyl acetate?Isopentyl acetate is a compound that is known as an alkanoate.
An alkanoate is a compound that is formed when an alkanol and an alkanoic acid react together, water is also produced.
The number of molecules in one gram of isopentyl acetate is determined from the molar mass of isopentyl acetate.
In one mole of isopentyl acetate, the number of molecules present is equal to the Avogadro number of molecules which is 6.02 * 10²³ molecules.
The molar mass of isopentyl acetate, C₇H₁₄O₂ = (12 * 7 + 1 * 14 + 16 * 2) g/mol
The molar mass of isopentyl acetate, C₇H₁₄O₂ = 130 g/mol
The number of moles in 1 g of isopentyl acetate, C₇H₁₄O₂ = 1/130
The number of moles in 1 g of isopentyl acetate, C₇H₁₄O₂ = 0.0077 moles
Number of molecules in 1 g of isopentyl acetate, C₇H₁₄O₂ = 0.0077 * 6.02 * 10²³
The number of molecules in 1 g of isopentyl acetate, C₇H₁₄O₂ = 4.21 * 10²¹ molecules.
Learn more about the number of molecules at: https://brainly.com/question/15379971
#SPJ1
Find the balance equation
_H2O+_O2=_H2O2
Answers
The balanced reaction equation of the reaction is; 2H2O + O2 → 2H2O2
How do we balance reaction equation?This is a chemical equation that represents the reaction between water (H2O) and oxygen (O2) to form hydrogen peroxide (H2O2). The coefficients in front of each substance indicate the relative number of molecules involved in the reaction.
Recall that we can balance the reaction equation by ensuring that the atoms of the elements on both sides of the reaction equation are the same as we have above.
Learn more about reaction equation:https://brainly.com/question/3588292
#SPJ1
Which answer best defines solute ?
Answers
Answer:
a substance that dissolves in another substance
Explanation:
a substance into which another substance dissolves
Answer:
sol·ute
noun
1.
the minor component in a solution, dissolved in the solvent.
Sodium (Na) atoms have a single valance electron, and so each of these Adam has only one electron to share. which of the following is true?
a. Sodium is stable and unreactive.
b. Sodium will share it’s one electron with hydrogen.
c. A molecule of sodium (Na2) will not exist in nature.
d. Sodium will share it’s one electron with seven other Atoms.
Answers
A molecule of sodium (Na2) will not arise in nature because sodium (Na) atoms only contain one valence electron. Since each of these Adam has only one electron to share, the true statement among the following is (c).
What are atoms called?We now understand that each atom is often composed of smaller particles, despite the fact that its original term referred to a particle that couldn't be further divided—the tiniest thing that was possible. Since they serve as the foundation for atoms, these particles are commonly referred to it as subatomic particles.
When was the first atom discovered?Democritus, a Greek philosopher, originally introduced the idea of the atom about 450 B.C. . Unfortunately, for more than 2000 years, the concept was essentially lost. John Dalton reintroduced the atom in 1800. He created the atomic theory and offered proof that atoms exist.
To know more about Atom visit:
https://brainly.com/question/1566330
#SPJ4
label the sun. which layer of the sun is #4?

Answers
Answer:
is D I guess; photosphere
Which element needs 3 valence electrons to complete its octet?
Answers
Answer: Gallium
Explanation:
Find a part of the article that describes signals that are sent within Diego’s body. Where does the signal come from, and how does it cause Diego to feel or react?
Answers
Answer:
The sensory receptors send signals to Diego's brain cells. These signals are messages that help Diego figure out what to do next. As Diego thinks, more signals move from one brain cell to another.
The sensory receptors send signals to Diego's brain cells. These signals are messages that help Diego figure out what to do next. As Diego thinks, more signals move from one brain cell to another.
What are sensory receptor?The capacity to react to stimuli is one of the traits of a living thing. The highly developed sensory system of humans can concurrently analyze thousands of incoming messages.
Dendrites of sensory neurons are specialized for receiving particular types of stimuli and are known as sensory receptors. There are three ways to classify sensory receptors.
Exteroceptors are located at or close to the skin's surface and are responsive to stimuli coming from the outside or the body's surface. These receptors include those for vision, hearing, smell, and taste as well as those for touch, pain, and temperature.
Therefore, The sensory receptors send signals to Diego's brain cells. These signals are messages that help Diego figure out what to do next. As Diego thinks, more signals move from one brain cell to another.
To learn more about sensory receptors, refer to the link:
https://brainly.com/question/3190796
#SPJ2
The equation of line t is y=–
1
7
x–2. Parallel to line t is line u, which passes through the point (7,6). What is the equation of line u?
Answers
The equation of line u, given that it is parallel to line t is:
y = –17x – 113
How to determine the equation of line uWe know that the equation of straight line is given as
y = mx + c
Where
y is the coordinate on y-axis x is the coordinate on x-axis m is the slope or gradient c is the intercept on y-axisFirst, we shall determine the slope of line u. This can be obtained as follow:
y = –17x – 2
Comparing y = mx + c with y = –17x – 2,
The slope (m₁) = –17
Recall,
The slope of parallel lines are equal.
Thus,
The slope of line u, is given as:
m₂ = m₁ = –17
Finally, we shall obtain the equation of line u as follow:
Coordinate = (7, 6) x coordinate 1 (x₁) = 7y coordinate 1 (y₁) = 6Slope of line u (m₂) = –17Equation of line u=?y – y₁ = m₂(x – x₁)
y – 6 = –17(x – 7)
Clear bracket
y – 6 = –17x – 119
Rearrange
y = –17x – 119 + 6
y = –17x – 113
Thus, the equation of line u is y = –17x – 113
Learn more about coordinate geometry:
https://brainly.com/question/20712656
#SPJ1
Answer:
Equation of line is given as y = mx + c, where m is the gradient and c is the y-intercept.
Parallel lines have the same gradient so gradient of line u is -17.
Equation of line u is y = -17x + c
Substitute (7,6) into the equation to find c.
6 = -17(7) + c
c = 125
Hence, equation of line u is y = -17x + 125.
Hydrogen reacts with oxygen according to the balanced equation
2H₂ (g) + O2(g) → 2H₂O(g). If X is the number of molecules of H₂ which react,
then the number of O2 molecules reacting is
Answers
Answer:
x/2
Explanation:
X = 2 molecules of H2
For 2 molecules of H2, there's only 1 molecule of O2. Meaning, there's twice the amount of H2, so O2 = x/2 molecules.
I hope I'm understanding this question right.