Answers
Answer:
No
Explanation:
not every species take care of their offspring/babies some abandon their babies and leave and some take care of them till they are older
Related Questions
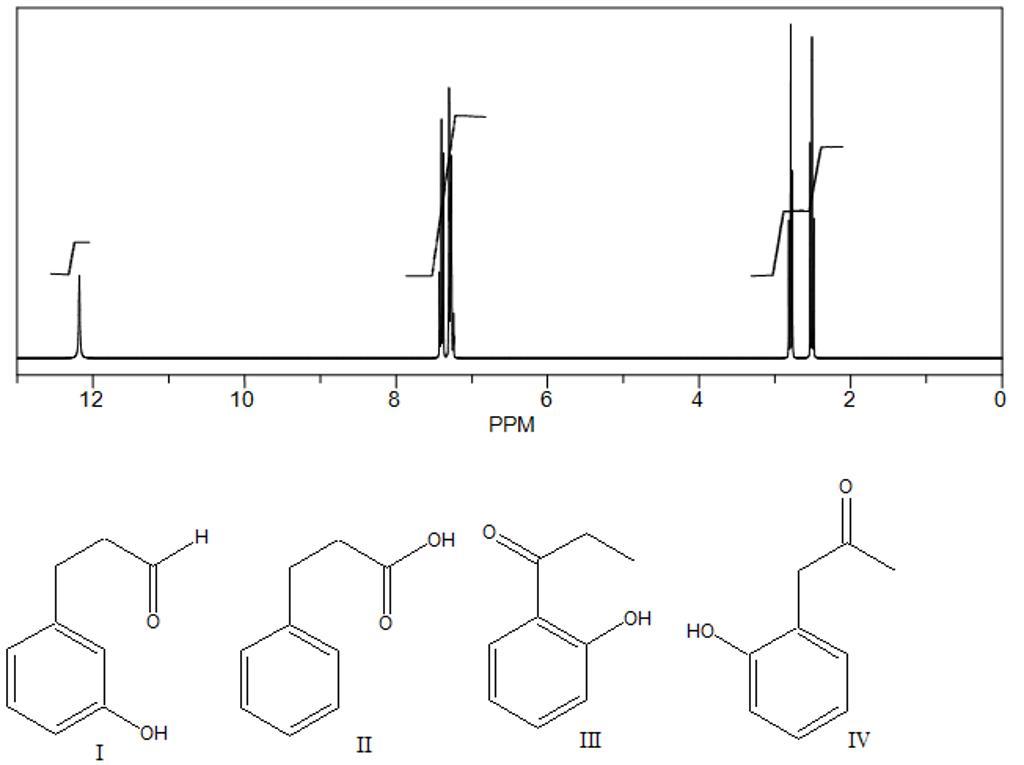
a compound with a molecular formula c9h10o2 has the following 1h nmr spectrum. which of the following structures is consistent with this spectrum?
Answers
structure II is correct answer
in NMR peak spectrum at 12 ppm is only for the carboxylic acid peak
in given options there is only one option with carboxylic acid functional group .therefore the answer is structure 2 is correct answer
Structures are compatible with this spectrum since no response would occur in the absence of rotation. The change in light intensity with regard to wavelength or frequency is referred to as a "spectrum." While a spectroscope is used to see spectra visually, a spectrograph is a device used to record or map spectrum.
Options and reference diagram are provided in below the solution.
Learn more about spectrum from here:
https://brainly.com/question/21134950
#SPJ4
What is the mass of 6.02 x 1024 molecules of the compound HCl?
Answers
Answer:
First, we need to determine the molar mass of HCl.
The molar mass of HCl = the mass of hydrogen (1.008 g/mol) + the mass of chlorine (35.45 g/mol) = 36.45 g/mol.
Next, we can use Avogadro's number (6.02 x 10^23 molecules/mol) to convert the number of molecules to moles:
6.02 x 10^24 molecules / 6.02 x 10^23 molecules/mol = 10 moles
Finally, we can use the molar mass to convert moles to grams:
10 moles x 36.45 g/mol = 364.5 grams
Therefore, the mass of 6.02 x 10^24 molecules of HCl is 364.5 grams.
:. It means 1 mole of Hcl
:. To find the mass of HcL
no of moles = mass/ molar mass
To get the molar mass of HCL {H=1 CL=35.5}
:. H+CL = 1+ 35.5 =36.5
So we have our molar mass and number of moles now
Then we input it in the eqn
1=x/36.5
X= 36.5g of HCl
What was the theoretical yield of a reaction if 50g are formed with a 10% yield?
A: 200g
B: 100g
C: 250g
D: 500g
Answers
Answer: 500g
Explanation:
We are asked to find the theoretical yield of a reaction, and we are given the following information:
In order to find the the theoretical yield we must use the following formula:
We have to convert the percents to real numbers before the calculations. We can do it dividing the percent value into 100, so:
"Enter your answer in the provided box. A person's blood alcohol (C2H5OH) level can be determined by titrating a sample of blood plasma with a potassium dichromate solution. The balanced equation is 16H+(aq) + 2Cr2O72−(aq) + C2H5OH(aq) → 4Cr3+(aq) + 2CO2(g) + 11H2O(l) If 35.46 mL of 0.05961 M Cr2O72− is required to titrate 28.40 g of plasma, what is the mass percent of alcohol in the blood?"
Answers
Answer:
0.1714 (w/w) %
Explanation:
Using the equation:
16H+(aq) + 2Cr2O72−(aq) + C2H5OH(aq) → 4Cr3+(aq) + 2CO2(g) + 11H2O(l)
2 moles of dichromate ion (Cr₂O₇²⁻) are used to titrate 1 mole of alcohol (C₂H₅OH)
35.46mL = 0.03546L of a 0.05961M Cr₂O₇²⁻ solution used to reach the equivalence point in the titration contains:
0.03546L ₓ (0.05961 moles Cr₂O₇²⁻ / L) = 2.114x10⁻³ moles Cr₂O₇²⁻
As 2 moles of dichromate reacts per mole of alcohol, moles of alcohol in the sample of plasma are:
2.114x10⁻³ moles Cr₂O₇²⁻ ₓ ( 1 mole C₂H₅OH / 2 moles Cr₂O₇²⁻) = 1.0569x10⁻³ moles of C₂H₅OH
As molar mass of alcohol is 46.07g/mol, mass of alcohol is:
1.0569x10⁻³ moles of C₂H₅OH ₓ (46.07g / mol) = 0.04869g of C₂H₅OH
Thus, mass percent of alcohol in the blood using the 28.40g of plasma is:
(0.04869g of C₂H₅OH / 28.40g) × 100 = 0.1714 (w/w) %
The mass percent of ethanol in the plasma has been 0.171%.
The titration has been the neutralization reaction, that converts the acid and base and results in the formation of the salt and water.
From the balanced equation, there has been the requirement of 2 moles of chromate for the neutralization of 1 mole of alcohol.
The moles of chromate in 0.05961 M has been:
Molarity = \(\rm \dfrac{Moles}{Volume}\)
Moles = Molarity × Volume
The moles of chromate has been:
Moles of chromate = 0.05961 mol × 0.03546 L
Moles of chromate = 0.002113 mol.
From the balanced equation:
2 moles chromate neutralizes = 1 mole of ethanol
0.002113 moles of chromate neutralizes = 0.00105 mol of Ethanol.
The mass of 0.00105 mol ethanol has been:
Mass = Moles × Molecular weight
Mass of 0.00105 mol of ethanol = 0.00105 mol × 46.07 g/mol
Mass of 0.00105 mol of ethanol = 0.0486 grams.
The total mass of plasma has been 28.40 grams.
The mass percent of ethanol in 28.40 grams plasma has been:
The mass percent of ethanol = \(\rm \dfrac{0.0486}{28.40}\) × 100
The mass percent of ethanol has been 0.171%.
The mass percent of ethanol in the plasma has been 0.171%.
For more information about mass percent, refer to the link:
https://brainly.com/question/5394922
What pressure will be exerted by 0.57 moles of CO, at a temperature of 298 K and a volume of 500ml?
Answers
Answer:
28atm
Explanation:
pv=nrt
p(0.500L)=(0.57mols)(0.08206 L * atm/ K mol)(298K)
p=(0.57mols)(0.08206 L * atm/ K mol)(298K)/(0.5L)
Just plug in numbers
27.8774232 ~ 28atm (2sig fig)
Express the number 4.80x10-1 in standard form
Answers
Calculate the standard entropy change
C2H2 (g) + 2H2 (g) → C2H6 (g
C2H2= 201
H2=131
C2H6 = 230
Answers
Entropy is a notion that essentially refers to the universe's propensity for chaos or the spontaneous changes that take place in everyday happenings. Here the standard entropy change for the given reaction is -233.
Entropy is typically referred to as a measurement of a system's randomness or disorder. In the year 1850, a German physicist by the name of Rudolf Clausius first proposed this idea. Entropy is a thermodynamic property that is used to characterize how a system behaves in terms of temperature, pressure, entropy, and heat capacity.
Here the standard entropy change is:
Entropy of products - entropy of reactants
ΔS = 230 - (201 + 2 ( 131)) = -233
To know more about entropy, visit;
https://brainly.com/question/17172535
#SPJ1
Use appropriate metric prefixes to write the following measurements without use of exponents.
Answers
An appropriate metric prefixes have been used to write the following measurements without use of exponents:
2.3 × 10⁻¹⁰ L = 0.23 nm.4.7 × 10⁻⁶ g = 4.7 μg.1.84 × 10² cm = 1.84 m.16.7 × 10⁶ s = 16.7 Ms.1.34 × 10⁻³ m = 1.34 mm.What is measurement?Measurement can be defined as an act or process through which the size, magnitude, dimensions, or distance traveled by a physical object or body is taken, especially for the purpose of an experiment.
What are metric prefixes?Metric prefixes are also referred to as SI prefixes and they can be defined as a series of prefixes to basic units of measurement, especially in the International System of Units (SI).
What is a conversion factor?A conversion factor can be defined as a number that is typically used to convert (change) a number in one (1) set of units to another, either by dividing or multiplying.
In Science, an appropriate conversion factor to an equal value must always be used when it is necessary to perform any mathematical conversion.
1 L = 1 × 10₉ nm
2.3 × 10⁻¹⁰ L = X nm
Cross-multiplying, we have:
X = 1 × 10₉ nm × 2.3 × 10⁻¹⁰ L
X = 0.23 nm
2.3 × 10⁻¹⁰ L = 0.23 nm.
1 g = 1 × 10⁶ μg
4.7 × 10⁻⁶ g = 4.7 μg.
Read more on conversion factor here: brainly.com/question/768963
#SPJ1
Complete Question:
Use appropriate metric prefixes to write the following measurements without use of exponents:
(a) 2.3 × 10⁻¹⁰ L
(b) 4.7 × 10⁻⁶ g
(c) 1.84 × 10² cm.
(d) 16.7 × 10⁶ s.
(e) 1.34 × 10⁻³ m
1a) Why do we study gases since reformation
Answers
We study gas since reformation in order to
Alter the the properties of hydrocarbon in the process of changing it's molecular structure.Its study also allows us to understand the behavior of matter.What are the properties of gases?Below are some few gas properties:
Diffusibility.CompressibilityExpansibilityLow DensityWhat is gas reformation?Gas reformation is defined production process which builds upon the existing natural gas.
Learn more about natural gas:
https://brainly.com/question/4677279
How many atoms are in 19.6 g of Sodium?
Answers
Answer:
Number of atoms = 5.1345 x 10²³
Explanation:
Given:
Mass of sodium = 19.6
Find:
Number of atoms
Computation:
We know that atomic mass of sodium is 22.98
So,
Number of atoms = [Mass of sodium/atomic mass of sodium]Avogadro's number
Number of atoms = [19.6 / 22.98]6.02 x 10²³
Number of atoms = 5.1345 x 10²³
can i have Brainliest i need 1 more
Nonane and 2,3,4-trifluoropentane have almost identical molar masses, but nonane has a significantly higher boiling point. Which of the following statements best helps explain this observation?
Answers
Compared to 2,3,4-trifluoropentane, the nonane's carbon chains are longer.
In chemistry, what exactly is a molar mass?A substance's molar mass is defined as its molecular weight in grams. By adding the molar masses of a substance's constituent atoms, we may get the substance's molar mass. Then, to convert between mass and the quantity of moles of the material, we may utilize the computed molar mass.
A molar mass is determined in what way?Adding the atomic masses of a particular substance results in the calculation of molar mass. Below each element's symbol on the periodic table is a designation of the mass of that specific element. The molar mass is obtained by averaging the atomic masses obtained from the periodic table.
To know more about Molar mass visit:
https://brainly.com/question/22997914
#SPJ1
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
the mass of electron is
Answers
Answer: is 9.1093837015 × 10−31 kg,
Explanation:
Answer: The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 1/1,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom.
Explanation:
good question but right and have's deteils
What is the correct conversion factor when converting from moles to liters?
Answers
The correct conversion factor when converting from moles to liters is the molar volume at a given temperature and pressure.
This value is dependent on the ideal gas law and can be determined using the ideal gas equation PV = nRT, where P represents pressure, V represents volume, n represents the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
The molar volume of an ideal gas at standard temperature and pressure (STP), which is 0 degrees Celsius (273.15 Kelvin) and 1 atmosphere (101.3 kilopascals), is approximately 22.4 liters per mole.
Therefore, to convert moles to liters, you can multiply the number of moles by the molar volume at STP, which gives you the volume in liters.
It's important to note that the molar volume is an approximation and assumes ideal gas behavior.
Additionally, if you are working with gases at different temperatures and pressures, you would need to use the appropriate molar volume value corresponding to the given conditions.
for such more questions on volume
https://brainly.com/question/29796637
#SPJ8
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8
15. Respiration is a three-step process that breaks down glucose and produces ATP. Describe each of the three steps.
Answers
Answer:
Cellular respiration uses energy in glucose to make ATP. Aerobic (“oxygen-using”) respiration occurs in three stages: glycolysis, the Krebs cycle, and electron transport.
Explanation:
In glycolysis, glucose is split into two molecules of pyruvate. This results in a net gain of two ATP molecules.
I WILL GIVE 35 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
The solution has a molarity of 0.0924 M.
What is molarity, for instance?The number of moles of solute per litre of solution is known as molarity.. For instance, water is both the solution and the solute when table salt is dissolved in it. Each mole of sodium chloride weighs 58.44 grammes. 58.44 grammes of sodium chloride are dissolved in one litre of water to produce one molar solution, or 1M.
Moles of solute per litre of solution is known as molarity (M).
Given: moles of NH3 = 0.355, volume of solution = 3.84 L
Molarity = 0.355 moles / 3.84 L = 0.0924 M
Therefore, the molarity of the solution is 0.0924 M.
To know more about molarity visit:-
https://brainly.com/question/8732513
#SPJ1
11) Methane and oxygen react to form carbon dioxide and water. What mass of water is formed if 0.80 g of methane reacts with 3.2 g of oxygen to produce 2.2 g of carbon dioxide
Answers
the mass of water formed is 1.8 g.
Which ONE of the following is an oxidation–reduction reaction? A) PbCO3(s) + 2 HNO3(aq) ––––> Pb(NO3)2(aq) + CO2(g) + H2O(l) B) Na2O(s) + H2O(l) –––> 2 NaOH(aq) C) SO3(g) + H2O(l) ––––> H2SO4(aq) D) CO2(g) + H2O(l) ––––> H2CO3(aq) E) C2H4(g) + H2(g) ––––> C2H6(g)
Answers
Answer:
E) C₂H₄(g) + H₂(g) ⇒ C₂H₆(g)
Explanation:
Which ONE of the following is an oxidation–reduction reaction?
A) PbCO₃(s) + 2 HNO₃(aq) ⇒ Pb(NO₃)₂(aq) + CO₂(g) + H₂O(l). NO. All the elements keep the same oxidation numbers.
B) Na₂O(s) + H₂O(l) ⇒ 2 NaOH(aq). NO. All the elements keep the same oxidation numbers.
C) SO₃(g) + H₂O(l) ⇒ H₂SO₄(aq). NO. All the elements keep the same oxidation numbers.
D) CO₂(g) + H₂O(l) ⇒ H₂CO₃(aq). NO. All the elements keep the same oxidation numbers.
E) C₂H₄(g) + H₂(g) ⇒ C₂H₆(g). YES. C is reduced and H is oxidized.
List the three pieces of evidence for sea floor spreading that also support the theory of continental drift
Answers
1. Same fossils have been found on different continents
2. Their coastlines fit together
3. Mountain ridges, land formations, glacial evidence, rocks…
Btw, can you describe the rotation of North America? I’m having trouble with continental drift too-
Aqueous hydrobromic acid HBr will react with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O. Suppose 3.24 g of hydrobromic acid is mixed with 3.0 g of sodium hydroxide. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant digits.
Answers
The maximum mass of water that could be produced by the chemical reaction is 1.35g.
What is a mole?
The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter.
A mole is defined as the amount of substance containing the same number of atoms, molecules, ions, etc. as the number of atoms in a sample of pure 12C weighing exactly 12 g.
Given,
The reaction - 2NaOH + 2HBr ⇒ 2NaBr + 2H₂O
Mass of HBr = 3.24g
Molar mass of HBr = 81
Mass of NaOH = 3g
Molar mass of NaOH = 40
Moles = mass ÷ molar mass
Moles of HBr = 3.24 ÷ 81 = 0.04 moles
Moles of NaOH = 3 ÷ 40 = 0.075 moles
The moles of NaOH is less, so NaOH is the limiting reagent and thus the mass of water would depend on the number of moles of NaOH.
From the reaction, 2 moles of NaOH give 2 moles of water
Therefore, 0.075 moles of NaOH will give 0.075 moles of water.
Mass of water = moles × molar mass
= 0.075 × 18
= 1.35g
Therefore, the maximum mass of water that could be produced by the chemical reaction is 1.35g.
Learn more about Moles, here:
https://brainly.com/question/20486415
#SPJ1
When you go up into the mountains or fly high in an airplane, the air is
thinner and the pressure is lower. The air pressure at sea level at a
temperature of 59°F (15°C) is equal to one atmosphere (Atm), and this
is the baseline reading for determining relative pressure.
HOW DOES THIS FACT( SUB QUESTION) RELATE TO MY RESEARCH QUESTION WHICH IS
What effect does the amount of air pressure have on how far a soccer ball travels when kicked?
Answers
The fact that air pressure is lower at higher altitudes or in airplanes is relevant to your research question because it means that the amount of air pressure can affect how far a soccer ball travels when kicked. In general, the higher the air pressure, the more force the air exerts on the soccer ball, which can cause it to travel a greater distance. Conversely, the lower the air pressure, the less force the air exerts on the soccer ball, which can cause it to travel a shorter distance.
Therefore, if you were to conduct a study on how the amount of air pressure affects the distance a soccer ball travels when kicked, you would need to take into account the fact that air pressure is lower at higher altitudes or in airplanes. This could be done by measuring the air pressure at the location where the soccer ball is kicked, and comparing it to the baseline air pressure at sea level to determine the relative air pressure. You could then use this information to calculate the expected distance the soccer ball should travel based on the amount of air pressure present. This would help ensure that your results are accurate and can be compared to other studies on this topic.
If you had 6H2 molecules and 4O2 molecules, how many H2O molecules could you produce?
Answers
Answer:
6
Explanation:
As , 2H2 + O2 = 2H2O
with 6 H2, 4O2 is excess.
H2O molecules formed = 6
In the molecule represented by the chemical formula H2SO4, there would be...Select one:a. 1 hydrogen atom.b. 2 hydrogen atoms.c. 2 sulfur atoms.d. 4 sulfur atoms.
Answers
2 hydrogen atoms.
Explanations:Given the chemical formula H2SO4,
The compound shows that the formula has 4 atoms of oxygen, one atom of sulfur and 2 hydrogen atoms.
Therefore, the number of hydrogen atom in the molecule H2SO4 is 2 hydrogen atoms.
When did Martin Luther king JR became a pastor/minister?
Answers
PLEASE HELP ME THERES 100 POINTS ON THE LINE


Answers
Answer:
1.93 2.86
Explanation:
exactly the reason for the above text
Which mineral might scratch the mineral fluorite, but would not scratch the mineral amphibole? 1 brucite 2. magnesite 3. carnallite 4. olivine
Answers
Answer:
olivine i think
Explanation:
A solution contains 1.817 mg of CoSO4 (155.0 grams/mole) per mL. Calculate the volume (in mL) of 0.009795 M Zn2 needed to titrate the excess complexing reagent after the addition of 70.00 mL of 0.009005 M EDTA to a 20.00 mL aliquot of the Co2 solution.
Answers
Answer:
85.952 ml \(Zn^2^+\) needed to titrate the excess complexing reagent .
Explanation:
Lets calculate
After addition of 80 ml of EDTA the solution becomes = 20 + 70 = 90 ml
As the number of moles of \(CoSO_4\) =\(\frac{Given mass }{molar mass}\)
=\(\frac{1.817}{155}\)
=0.01172
Molarity = \(\frac{no. of moles}{volume of solution}\)
=\(\frac{0.01172}{20}\)
=0.000586 moles
Excess of EDTA = concentration of EDTA - concentration of CoSO4
= 0.009005 - 0.000586
= 0.008419 M
As M1V1 ( Excess of EDTA ) = M2V2 \((Zn^2^+)\)
\(0.008419\times100ml=0.009795\times V2\)
\(V2=\frac{0.008419\times100}{0.009795}\)
V2 =85.952 ml
Therefore , 85.952 ml \(Zn^2^+\) needed to titrate the excess complexing reagent .
Lewis Structure for NO3-
Answers
Answer::
Explanation::
the atomic number of f plus the atomic number of Na equals the atomic number of this element
Answers
Answer:
Atomic no. of Fluorine =9
Atomic no. of Na=11
Therefore,
Atomic no. of x element=9+11=20
Therefore
,Element=Calcium