draw the lewis structure of iodine trifluoride, if3. do not include brackets or formal charges. if the molecule exhibits resonance, draw only one (1) structure. do not draw the other resonance forms.
Answers
Each atom's octet and the hydrogen molecule's duet are depicted in the Lewis structure in a full manner.
The Lewis structural rule is what.
Only one bond may be formed by hydrogen atoms, and they are always found at the extremities of a chain of atoms. Carbon, nitrogen, or oxygen are frequently bonded with hydrogen. With the exception of O2, O3, superoxides, and peroxides, oxygen atoms typically do not connect to one another.
Describe Lewis's structure with an example.
Lewis structure illustration
The octet rule, which states that atoms share electrons so that each has eight in its outer shell, is the foundation of a Lewis structure. For instance, the outermost electron of an oxygen atom possesses six protons.
To know more about lewis structure visit:
https://brainly.com/question/4144781
#SPJ4
Related Questions
how much of 6% saline solution should kent mix with 70 cubic centimeters of a 16% saline solution to produce 11% saline solution
Answers
70 cubic centimeters of 6% saline solution should kent mix with 70 cubic centimeters of a 16% saline solution to produce 11% saline solution
To determine the amount of a 6% saline solution that should be mixed with 70 cubic centimeters of a 16% saline solution to obtain an 11% saline solution, we can use a mixture equation based on the principle of conservation of mass.
Let's assume x represents the volume of the 6% saline solution to be added in cubic centimeters.
The amount of salt (in grams) in the 16% saline solution is given by:
Amount of salt in 16% solution = 70 cubic centimeters * 0.16 (salt concentration)
The amount of salt (in grams) in the 6% saline solution to be added is given by:
Amount of salt in 6% solution = x cubic centimeters * 0.06 (salt concentration)
The total amount of salt (in grams) in the resulting 11% saline solution is given by:
Amount of salt in resulting solution = (70 + x) cubic centimeters * 0.11 (salt concentration)
According to the principle of conservation of mass, the amount of salt in the initial solution must be equal to the amount of salt in the resulting solution. Therefore, we can set up the following equation:
Amount of salt in 16% solution + Amount of salt in 6% solution = Amount of salt in resulting solution
70 * 0.16 + x * 0.06 = (70 + x) * 0.11
11.2 + 0.06x = 7.7 + 0.11x
0.11x - 0.06x = 11.2 - 7.7
0.05x = 3.5
x = 3.5 / 0.05
x = 70
Therefore, Kent should mix 70 cubic centimeters of the 6% saline solution with the 70 cubic centimeters of the 16% saline solution to produce an 11% saline solution.
To know more about saline solution here
https://brainly.com/question/32838167
#SPJ4
Please help! Please!!!
Look at the picture down below to answer the question!
>>Select the 2 that apply.
Q: An unsaturated hydrocarbon is represented by which structural formula(s)?
>>Choose two from the picture below that matches with the question above!

Answers
Answer:
A and D
Explanation:
unsaturated hydrocarbons have double or triple bonds
when a solvent has dissolved all the solute it can at a particular temperature, it is said to be
Answers
The solvent is said to be saturated when it has dissolved all the solute it can at a particular temperature.
The Significance of Saturation in Solvent-Solute InteractionsThe relationship between solvents and solutes is an important one in the study of chemistry. In order to understand the dynamics of this relationship, it is important to understand the concept of saturation. When a solvent has dissolved all the solute it can at a particular temperature, it is said to be saturated. In this essay, the significance of saturation in solvent-solute interactions will be discussed in detail.
At the most basic level, saturation is important because it helps to inform chemists of the maximum amount of solute that can be dissolved in a solvent at any given temperature. This information can be used to control the concentration of a solution and to ensure that it is within the desired range for a particular application.
Learn more about temperature:
https://brainly.com/question/20595779
#SPJ4

Difference between atomic number and atomic weight any 2 points
Answers
Answer:
According to the definitions given, atomic mass which is also called atomic weight is the measured total mass of an element's atom. Whereas, atomic number is nothing but the total number of neutrons and protons in the nucleus of an atom....
can u talk to in comment section
This is a question of 11 grade chemistry, what I have learned and should applied on this question is the mole and stoichiomestry. Please help me solving this.

Answers
The substance that contains the greatest amount (in moles) of carbon atoms per mole of compound is benzoyl peroxide (\(C_1_4H_1_0O_4).\)
Option D is correct
How do we calculate?We analyze each substance by:
A. Aspirin (C9H8O4)
Molar mass of carbon (C) = 12.01 g/mol
Number of moles of carbon atoms in aspirin = 9
Caffeine (C8H10N4O2)
Molar mass of carbon (C) = 12.01 g/mol
Number of moles of carbon atoms in caffeine = 8
Saccharin (C7H5NO3S)
Molar mass of carbon (C) = 12.01 g/mol
Number of moles of carbon atoms in saccharin = 7
. Benzoyl peroxide (C14H10O4)
Molar mass of carbon (C) = 12.01 g/mol
Number of moles of carbon atoms in benzoyl peroxide = 14
Carbon tetrachloride (CCl4)
Molar mass of carbon (C) = 12.01 g/mol
Number of moles of carbon atoms in carbon tetrachloride = 1
Learn more about benzoyl peroxide at:
https://brainly.com/question/30589244
#SPJ1
Combustion reactions typically involve all of the following except
a. The consumption of oxygen gas
b. The production of a Hydrocarbon
c. The production of water
d. The release of carbon dioxide
Answers
Answer:
b. The production of a HydrocarbonExplanation:
In Combustion reaction usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water.
2) Check all that apply.
What are the three major groups of elements?
Answers
Answer:
metals, nonmetals and metalloids
Explanation:
2. Nonmetals
3. Metalloids
The specific heat of silver is 0.24 cal/goC. How many calories of energy are needed to warm 4.37 g of silver from 25.0 oC to 27.5 oC
Answers
The amount of energy needed to warm 4.37 g of silver from 25.0 oC to 27.5 oC is approximately 2.61 calories. This is calculated using the formula Q = m * c * ΔT.
To calculate the amount of energy needed to warm a substance, we can use the formula:
Q = m * c * ΔT
where Q is the energy in calories, m is the mass in grams, c is the specific heat in cal/goC, and ΔT is the change in temperature in oC.
In this case, we need to calculate the energy required to warm 4.37 g of silver from 25.0 oC to 27.5 oC. The mass of the silver is 4.37 g, the specific heat is 0.24 cal/goC, and the change in temperature is 27.5 oC - 25.0 oC = 2.5 oC.
Using the formula, we can calculate:
Q = 4.37 g * 0.24 cal/goC * 2.5 oC ≈ 2.61 calories
Therefore, approximately 2.61 calories of energy are needed to warm 4.37 g of silver from 25.0 oC to 27.5 oC.
To learn more about specific heat click here: brainly.com/question/31608647
#SPJ11
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
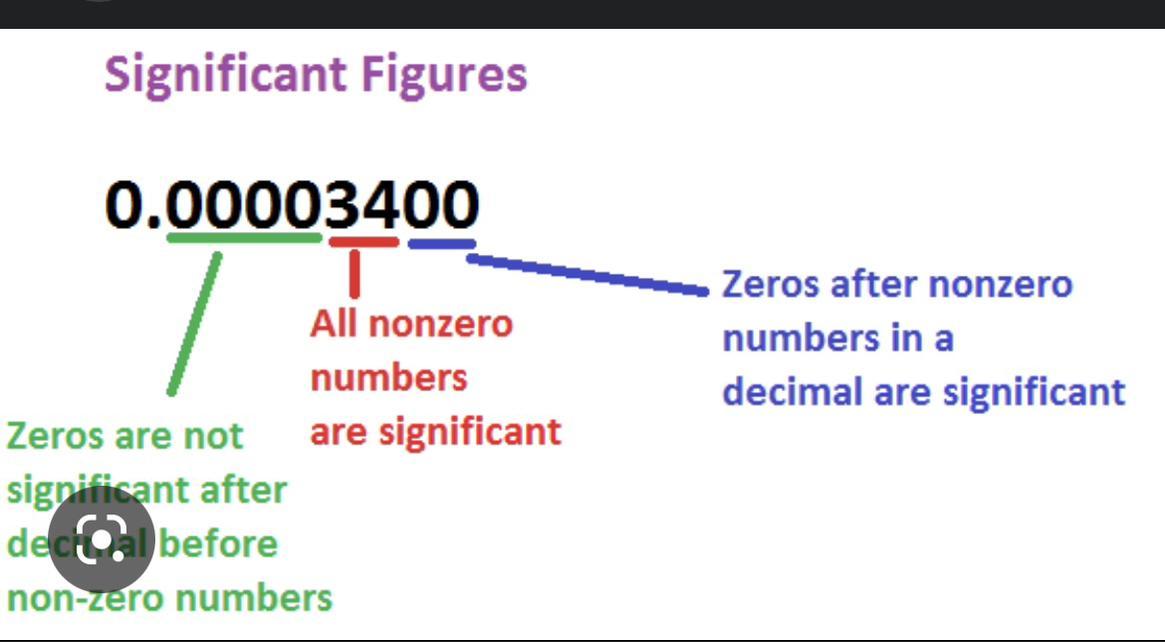
what is the number of moles of solute in 250 mL of a .80M solution
Answers
Answer:
There are 0.1 moles of solute in 250 mL of 0.4 M solution
Explanation:
because it is
QUESTION 4 [5 MARKS] Table 5 (a) Assume the consumption function takes the form \( \mathrm{C}=\mathrm{Ca}+(\mathrm{c}) \mathrm{Y} \), then the consumption function based on the information in Table 5
Answers
The consumption function based on the information in Table 5 is as follows: C = 2577 + 0.75Y. It is given, Consumption function, C = Ca + cY Where, Ca is autonomous consumption expenditure, c is marginal propensity to consume (MPC)Y is disposable income
The consumption function based on the information in Table 5 is: Table 5Income(¥ billions)
Consumption(¥ billions)100025020007526000102772750120301.
Write the consumption function in the given format. Ca = Autonomous consumption expenditure c = MPCY = Disposable Income Calculation:
We can obtain the value of Ca as follows: C = Ca + cY
Put the given values, C = 2577Ca + 0.75YAt Y = 1000 billion, C = 2577(1) + 0.75(1000)
= 8327 billion
At Y = 2000 billion, C = 2577(1) + 0.75(2000)
= 13277 billion
At Y = 3000 billion, C = 2577(1) + 0.75(3000)
= 18277 billion
At Y = 4000 billion, C = 2577(1) + 0.75(4000)
= 23277 billion
At Y = 5000 billion, C = 2577(1) + 0.75(5000)
= 28277 billion
Therefore, the consumption function based on the information in Table 5 is as follows: C = 2577 + 0.75Y.
To know more about consumption function, refer
https://brainly.com/question/28145641
#SPJ11
does the mean molecular speed chnage as much as the temperature as the water heats up? explain please help
Answers
Explanation:
When water is heated up then its molecules move rapidly from one place to another. Hence, kinetic energy of the molecules tend to increase.
Also, K.E = \(\frac{3}{2}kT\)
where, T = temperature
Therefore, more is the increase in temperature more will be the mean molecular speed change in the water molecules.
As a result, mean molecular speed change increases with increase in temperature and vice-versa.
Write a Lewis structure that obeys the octet rule for each molecule or ion. Include resonance structures if necessary and assign formal charges to each atom.
Answers
Answer:
A structure that obeys the octet rule for each molecule or ion is \(XeO_4\)
Explanation:
Here in \(XeO_4\) , Xenon has 8 valence electrons and each oxygen atom has 6 valence electrons
Lewis structure for \(XeO_4\) is shown below :
Here , all atoms are having their complete octet .
All the atoms in this Lewis structure is having their complete octet .
Resonance structure is not required as all atoms are same that is oxygen .

Help!!
What is the concentration of an unknown with an absorbance of 0.4 using the plot?

Answers
Answer:3,4,1,2
Explanation:
hope this helped
E2-13 (Identification of funds) For each of the following situations, indicate which fund would be used to report the transaction. Note: Several of the transactions require using more than one fund.
Answers
The funds that would be used to report the transactions are:
a. General Fund
b. General Fund
c. General Fund, Special Revenue Funds, and Capital Projects Funds
d. General Fund and Debt Service Fund
e. General Fund and Capital Projects Fund
f. Special Revenue Funds
g. Special Revenue Funds
h. Capital Projects Funds
i. Capital Projects Funds
j. Capital Projects Funds
k. Debt Service Fund
l. Debt Service Fund
m. Debt Service Fund
n. Debt Service Fund
a. City Council adopts a $5,000,000 budget for the General Fund, which includes $150,000 for contingencies.
The General Fund is the appropriate fund to report the transaction.
b. City purchases $100,000 of supplies for the General Fund.
The General Fund is the appropriate fund to report the transaction.
c. City pays $4,000 for its share of an annual audit performed for the General Fund, Special Revenue Funds, and Capital Projects Funds.
The General Fund, Special Revenue Funds, and Capital Projects Funds are the appropriate funds to report the transaction.
d. City levies property taxes of $9,000,000 to be distributed as follows: $6,000,000 to the General Fund and $3,000,000 to the Debt Service Fund.
The General Fund and Debt Service Fund are the appropriate funds to report the transaction.
e. City transfers $50,000 from the General Fund to the Capital Projects Fund.
The General Fund and Capital Projects Fund are the appropriate funds to report the transaction.
f. City receives a federal grant of $400,000 for the acquisition of equipment. The grant must be used for that purpose or returned to the federal government.
Special Revenue Funds are the appropriate funds to report the transaction.
g. City receives a bequest of $100,000 that is to be used to provide scholarships for needy children.
Special Revenue Funds are the appropriate funds to report the transaction.
h. City receives a donation of land worth $50,000 that is to be used for a park.
Capital Projects Funds are the appropriate funds to report the transaction.
i. City spends $200,000 on the construction of a new street that will be financed by the issuance of general obligation bonds.
Capital Projects Funds are the appropriate funds to report the transaction.
j. City issues $500,000 of general obligation bonds to finance the construction of a new community center.
Capital Projects Funds are the appropriate funds to report the transaction.
k. City establishes a debt service fund to accumulate resources for the payment of future interest and principal on the bonds authorized in transaction (j).
Debt Service Fund is the appropriate fund to report the transaction.
l. City repays $50,000 of the principal on the bonds issued in transaction (j).
Debt Service Fund is the appropriate fund to report the transaction.
m. City pays the semiannual interest on the bonds issued in transaction (j).
Debt Service Fund is the appropriate fund to report the transaction.
n. City pays $2,000 for administrative costs associated with the issuance of the bonds authorized in transaction (j).
Debt Service Fund is the appropriate fund to report the transaction.
Learn more about transactions
https://brainly.com/question/24730931
#SPJ11
FOR LondonCreamCakes
no sure if its right but im trying to help.. lol dont mind all the tabs

Answers
Answer:
It kinda helps but not really
Thanks for trying anyway doe!
Explanation:
the zinc in a 1.441g sample of foot powder was precipitated as znnh4po4. strong heating of the precipitate yielded 0.4089 g zn2p2o7. calculate the mass percent of zinc in sample of foot powder
Answers
The mass percent of zinc in sample of foot powder is 12.176%.
Molecular weight of Zn₂P₂O₇ is 304.7033 g.
weight of zinc in Zn₂P₂O₇=2×65.38=130.76 g as there are 2 zinc atoms.
As 304.703 g Zn₂P₂O₇ contains 130.76 g zinc
∴0.4089 g sample contains , 0.4089×130.76/304.703=0.17547 g zinc
As the sample was precipitated ,1.441 g sample contains 0.17547 g of zinc
The mass percent of zinc in the sample is 0.17547/1.441×100=12.176%
What are mass percent calculations ?It is a way of obtaining concentration in a mixture .It shows the mass of solute which is present in a given mass of solution . It is expressed in terms of mass or moles.It is a simple percentage of a component.
Learn more about mass percent calculations here:
https://brainly.com/question/5840377
#SPJ1
Calculate the volume of a solution of 3.6 M NH4Cl needed to prepare 350.0 mL of 1.24M NH4CH
Select one:
a. 120 mL
b. 12.0 mL
c. 967 ml
d. 96.7 ml
How many grams of ammonium chloride are in the solution you created in the above problem Select one:
a. 23.28
b. 0.438
c 53.58
d. 232 23
Answers
Answer:
select one :c
select two:d
Se mezclan por error 70 mililitros de solución de HCl 0.5 Normal con 250 mililitros de solución de hidróxido de sodio 0.30 normal. Calcular: El volumen final de la solución resultante El carácter de la solución resultante La concentración final de la solución resultante El pH de la solución resultante El valor del pOH de la solución resultante
Answers
Answer:
asxrsse367721479009qqucinübjpnpnfzdh ilo h
If you wanted to mix pure methane with water and end up with 90 gallons of 60% methane, how many gallons of each should you use?
You should use ________ gallons of water and _________ gallons of methane
Answers
To determine the amount of water and methane needed, we can set up a system of equations based on the desired composition of the mixture. you should use 36 gallons of water and 54 gallons of methane to obtain a mixture of 90 gallons with a methane concentration of 60%.
Let's assume x represents the number of gallons of water and y represents the number of gallons of methane. We have the following information: The total volume of the mixture is 90 gallons: x + y = 90. The mixture should be 60% methane: (y / (x + y)) * 100 = 60. Simplifying the second equation: y / (x + y) = 0.6. Now we can solve the system of equations: From equation 1, we can express x in terms of y: x = 90 - y. Substituting this into equation 2: y / ((90 - y) + y) = 0.6. Simplifying further: y / 90 = 0.6. Solving for y: y = 0.6 * 90. y = 54. Now we can find x using equation 1: x = 90 - y. x = 90 - 54. x = 36. Therefore, you should use 36 gallons of water and 54 gallons of methane to obtain a mixture of 90 gallons with a methane concentration of 60%.
To learn more about methane, https://brainly.com/question/31473733
#SPJ11
Santee Cooper is stocking lakes Marion and Moultrie with 109,000 sterile grass carp. The grass carp, which eat hydrilla (Invasive species), are the most effective and least Intrusive means to control the weed. What type of control are they using?
A Invasive
B biological
C.chemical
D physical
Answers
Answer:
biological
Explanation:
i took the test and got it right
1. Click on this link to open a simulation on greenhouse gases. In the third box on
the right titled "Atmosphere during..." Click on today and then click on 1750.
What is the CO₂ level today and was the CO₂ level in 1750? When CO₂ dissolves
in water it creates Carbonic Acid. What would happen to the pH of the Great
Lakes (go up or down) as more CO₂ enters the atmosphere/ lakes?
Answers
Based on the data from the Mauna Loa Observatory, the atmospheric CO₂ concentration was around 280 ppm in 1750 but the atmospheric CO₂ concentration today is 415 ppm.
The pH of the Great Lakes would go down as more CO₂ enters the atmosphere/ lakes.
What would happen to the pH of the Great Lakes as more CO₂ enters the atmosphere/ lakes?When CO₂ dissolves in water, it reacts with water molecules to form carbonic acid, which increases the acidity, thus lowering the pH of the water.
The equation of the reaction is given below:
CO₂ + H₂O ---> H₂CO₃The Great Lakes are freshwater bodies having an alkaline pH between 7.0 and 8.5.
As more CO₂ enters the atmosphere and dissolves in the lakes, it will increase the concentration of carbonic acid and decrease the pH of the water.
Learn more about pH at: https://brainly.com/question/172153
#SPJ1
List the following types of electromagnetic radiation in order of increasing wavelength. Rank the following types of electromagnetic radiation from lowest to highest wavelength. To rank items as equivalent, overlap them. radio waves, microwaves, infrared radiation, ultraviolet radiation
Answers
Electromagnetic radiation can be classified into different types based on their wavelengths. The types of electromagnetic radiation you've mentioned can be ordered from the lowest to the highest wavelength as follows:
1. Ultraviolet radiation
2. Infrared radiation
3. Microwaves
4. Radio waves
Ultraviolet radiation has the shortest wavelength among these types, ranging from approximately 10 to 400 nanometers (nm). It is responsible for skin tanning and can be harmful if the exposure is excessive, potentially causing sunburn or even skin cancer.
Next in the order is infrared radiation, with wavelengths ranging from about 700 nm to 1 millimeter (mm). Infrared radiation is emitted by warm objects, and it plays a significant role in heat transfer and remote sensing technologies.
Microwaves have a broader wavelength range than infrared radiation, from approximately 1 mm to 100 centimeters (cm). They are commonly used in various communication systems, as well as in heating food in microwave ovens.
Finally, radio waves have the longest wavelength in this list, ranging from around 1 cm to 100 kilometers (km). They are widely used for communication purposes, such as in television and radio broadcasting, mobile phones, and satellite communication.
Learn more about wavelengths here :-
https://brainly.com/question/6916860
#SPJ11
Which of these elements is found in a family with the above electron configuration?
f Sr g Sb h Al j Si
Answers
Sr is the only element among the given options that belongs to a family with the above electron configuration, specifically the alkaline earth metal family.
Explain these elements and found in a family with the above electron configuration?f Sr g Sb h Al j SiAmong the given elements, only Sr (strontium) has an electron configuration that belongs to a family. Elements within the same family have similar chemical and physical properties due to the same number of valence electrons.
Strontium belongs to the alkaline earth metal family, which is the second column of the periodic table. The electron configuration of strontium is [Kr] 5s2, meaning it has two valence electrons in the outermost shell. Other elements in the alkaline earth metal family, such as magnesium and calcium, also have two valence electrons and share similar properties with strontium.
Sb (antimony), Al (aluminum), and Si (silicon) are not in the same family as Sr, as they have different electron configurations and belong to different columns in the periodic table. Sb belongs to the metalloid family, Al is a member of the boron family, and Si belongs to the carbon family.
In summary, Sr is the only element among the given options that belongs to a family with the above electron configuration, specifically the alkaline earth metal family.
Learn more about electron configuration
brainly.com/question/31812229
#SPJ11
Unlike bacteria, an animal cell contains
Answers
Answer:
Membrane
Explanation:
Unlike a bacteria , an animal cell contains membrane which bounds organelles.
Explain why Noble Gases (group 18 elements) have little/no electronegativity
Answers
Answer: they don't have values there because they aren't on the pauling scale of electronegativity, as they don't form any compounds with other elements. However, argon and neon can technically form compounds with other elements; it is just extremely unlikely.
Explanation:
Argon (Ar): (Ne) 3s2 3p6
O longhand notation
O noble-gas notation
Answers
Answer:
It is Noble-gas notation
Explanation:
Just did it on Edgenuity 2020
Two or more than two atoms with different physical or chemical properties can not combine together to form an element. Therefore, the given electronic configuration represents noble-gas notation.
What is element?Element generally consist of atoms or we can atoms combine to form element. Atoms of an element is always same, means all the properties of all atoms of one type of element is same.
The systematic distribution of electrons in the various atomic orbitals is called its electronic configuration. The atomic number of argon is 18. The electronic configuration of argon is (Ne) 3s² 3p⁶. 1,2,3 represents the number of shells and s and represents the orbitals. The superscripts represents the number of electrons in each orbitals. The given electronic configuration represents noble-gas notation.
Therefore, the given electronic configuration represents noble-gas notation.
To know more about element, here:
brainly.com/question/8460633
#SPJ2
A ruby laser produces red light that has a wavelength of 5.00 × 10^-7 m. Calculate its energy in joules
Answers
Answer:
The energy of a ruby laser that produces red light with a wavelength of 5.00 × 10^-7 m is 3.98 × 10^-19 J.
Explanation:
The energy of a photon is given by the formula E = hf where h is Planck’s constant and f is the frequency of the photon. The frequency of the photon can be calculated using the formula f = c/λ where c is the speed of light and λ is the wavelength of the photon.
Substituting the given values, we get:
f = c/λ = 3.00 × 10^8 m/s / 5.00 × 10^-7 m = 6.00 × 10^14 Hz
E = hf = (6.626 × 10^-34 J s) × (6.00 × 10^14 Hz) = 3.98 × 10^-19 J
Therefore, the energy of a ruby laser that produces red light with a wavelength of 5.00 × 10^-7 m is 3.98 × 10^-19 J.
Hello again!! :)
please answer these about Charles law


Answers
Answer:
1. V2.
2. 299K.
3. 451K
4. 0.25 x 451 = V2 x 299
Explanation:
1. The data obtained from the question include:
Initial volume (V1) = 0.25mL
Initial temperature (T1) = 26°C
Final temperature (T2) = 178°C
Final volume (V2) =.?
2. Conversion from celsius to Kelvin temperature.
T(K) = T (°C) + 273
Initial temperature (T1) = 26°C
Initial temperature (T1) = 26°C + 273 = 299K
3. Conversion from celsius to Kelvin temperature.
T(K) = T (°C) + 273
Final temperature (T2) = 178°C
Final temperature (T1) = 178°C + 273 = 451K
4. Initial volume (V1) = 0.25mL
Initial temperature (T1) = 299K
Final temperature (T2) = 451K
Final volume (V2) =.?
V1 x T2 = V2 x T1
0.25 x 451 = V2 x 299
Select the correct answer.
The table shows the specific heat of four substances—brick, dry soil, paper, and water. If all four substances were exposed to sunlight for the same amount of time, which substance would heat up the slowest?
brick 0.9
dry soil 1.26
paper 1.336
water 4.18
A.
water
B.
dry soil
C.
paper
D.
brick
Answers
If all four substances were exposed to sunlight for the same amount of time, brick is the substance that heats up the slowest. Option D is correct.
The certain heat of brick is 0.9, which specifies that it needs less heat energy to increase its temperature compared to the other substances listed
Particularly, brick has a lower heat size, meaning it can engross less heat energy per unit mass. Accordingly, when exposed to sunlight, the brick will heat up in proportion slowly compared to the other substances.
So, the substance that would heat up the slowest when exposed to sunlight for the same duration is brick.
Learn more about substances here:
https://brainly.com/question/32499949