energized electrons extracted during the redox reaction of cell respiration are transported to the cell using which of the following coenzymes
Answers
Protons are pumped into the intermembrane gap as the electrons pass along the electron transport chain. These protons' concentration changes when they flow back down.
To what do electrons move during cellular respiration?The electron transport chain, a collection of proteins in the inner membrane of the mitochondrion, is where the electron carriers deliver the electrons. The electron transport chain is where electrons go from a higher to a lower energy level before being transferred to oxygen (forming water).
Is NADH used in cellular respiration to transport electrons?NADH: A high-energy electron carrier that carries electrons produced during the Krebs Cycle and glycolysis to the electron transport chain.
To know more about cellular respiration visit:-
https://brainly.com/question/13721588
#SPJ4
Related Questions
true or false:
1. chemical bonds have only one form.
2. All compounds are neutral.
3. covalent bonds do not exhibit any sort of preferential charge between atoms.
4. a covalent bond can only involve one shared electron.
Answers
Match the structural formulas given to you below with the correct chemical formula from the bank above. (Image provided)

Answers
1) C3H6O because there are 6 hydrogen in formula and one oxygen. 2) H2So4 sulphric acid.
What three categories exist for chemical formulas?Chemical formulas can be divided into three categories: empirical, molecular, and structural. Molecular formulas display the quantity of each type of atom in a molecule, while structural formulas display the simplest whole-number relationship between the atoms in a compound. Empirical formulas display the simplest whole-number relationship between the atoms in a compound.
3) C2H2 ethyne where carbons have triple bond.
4) CO carbon monoxide where C and O have triple bond between them .
5) HNO3 is nitric acid .
6) CH2F2
7) Ch2O
8) C2H4 is ethene in which carbon carbon have double bonds.
9) SO3 sulphur trioxide.
10) CH3F
To learn more about chemical formula refer
https://brainly.com/question/11995171?
#SPJ4
Assign oxidation numbers to each atom in the following compounds or ions.
a. HF d. P I 3 g. H2C O 3
b. C I 4 e. C S 2 h. NO -2
c. H 2O f. N a 2O 2i. SO 2-
Answers
In all compounds, hydrogen has an oxidation number of +1. In compounds with oxygen, oxygen usually has an oxidation number of -2. There are a few exceptions to these rules, but they are relatively rare. For example, in peroxides, oxygen has an oxidation number of -1.
Oxidation numbers are assigned based on a set of rules, and they represent the hypothetical charge an atom would have if all shared electrons were assigned to the more electronegative atom.
a. HF:
The oxidation number of hydrogen (H) is +1.
The oxidation number of fluorine (F) is -1.
b. CI₄:
The oxidation number of carbon (C) is +4.
The oxidation number of iodine (I) is -1.
c. H₂O:
The oxidation number of hydrogen (H) is +1.
The oxidation number of oxygen (O) is -2.
d. PI₃:
The oxidation number of phosphorus (P) is +3.
The oxidation number of iodine (I) is -1.
e. CS:
The oxidation number of carbon (C) is +4.
The oxidation number of sulfur (S) is -2.
f. Na₂O₂:
The oxidation number of sodium (Na) is +1.
The oxidation number of oxygen (O) is -1.
g. H₂CO₃:
The oxidation number of hydrogen (H) is +1.
The oxidation number of carbon (C) can vary, but in this case, it is +4.
The oxidation number of oxygen (O) is -2.
h. NO²⁻:
The oxidation number of nitrogen (N) is +3.
The oxidation number of oxygen (O) is -2.
i. SO²⁻:
The oxidation number of sulfur (S) is +4.
The oxidation number of oxygen (O) is -2.
To know more about the Oxidation numbers refer here,
https://brainly.com/question/29100691#
#SPJ11
Cu2(s)+O2(g)=Cu2O(g)+SO2(g)
please help urgent will give brainiest
Answers
Answer:
2 Cu2S + 3 O2 = 2 Cu2O + 2 SO2
Explanation:
2 Cu2S + 3 O2 = 2 Cu2O + 2 SO2
I need help with this. Thank youuuuuuu

Answers
The number of atoms in 3 moles K of the particle is 1.806 x 10²⁴ atoms.
What is the number of atoms in the given moles?
The value of one mole of an atom is equal to exactly 12 grams of pure carbon-12.
12.00 g (C-12 ) = 1 mol C-12 atoms = 6.02 × 10²³ atoms .
The number of particles in 1 mole is called Avogadro's Number (6.0221421 x 10²³).
The number of atoms in 3 moles K of the particle is calculated as follows;
= 3 moles x 6.02 × 10²³ atoms / mol
= 1.806 x 10²⁴ atoms
Learn more about number of atoms here: https://brainly.com/question/6258301
#SPJ1
Methyl isocyanate is used in the industrial synthesis of a type of pesticide and herbicide known as a carbamate. As a historical note, an industrial accident in Bhopal, India in 1984 resulted in leakage of an unknown quantity of this chemical into the air. An estimated 200,000 persons were exposed to its vapors and over 2000 of these people died.
Methyl isocyanate reacts with strong acids, such as sulfuric acid, to form a cation. For protonation on O , which resonance contributor is most important?
Answers
Protonation of isocyanate happens on Oxygen atom. Here the nitrogen atom attached to the carbocation is the resonance contributor
Methyl isocyanate is a precursor or intermediate in the production of carbamate, which is a popular pesticide and herbicide. Protonation happens in the carbenium ion, or carbocation, since it is sp hybridized with oxygen and nitrogen.( Image is given below)
There are two contributing resonance structures in the image. The protonation of nitrogen leads to a formation of resonance structure with triple bond on oxygen, which is very unlikely. The other structure contributes to the formation of protonated product, since triple bonded nitrogen atom is very likely to form.
So nitrogen is the resonance contributor for the protonation of oxygen.
For more information regarding resonance, kindly refer
https://brainly.com/question/29547999
#SPJ4

for a solution equilibrium, a change in concentration of a reactant or product does not change keq. group of answer choices true false
Answers
The given statement "A change in the concentration of the reactant or the product do not change keq" will be false. Because a change in the concentration of a reactant or product will change the value of Keq.
According to Le Chatelier's principle, when a stress is applied to a system at equilibrium, the system will respond in a way that partially counteracts the stress and reestablishes equilibrium. Changes in the concentration of a reactant or product will alter the concentrations of all species present in the reaction, and thus will disturb the equilibrium.
For example, if the concentration of a reactant is increased, the reaction will shift towards the product side to partially counteract the increase in the concentration of the reactant. This will result in an increase in the concentration of products and a decrease in the concentration of reactants. As a result, the value of Keq will change to reflect the new equilibrium concentrations of reactants and products.
To know more about Le Chatelier's principle here
https://brainly.com/question/2001993
#SPJ4
Atoms are made up of?
Answers
Answer:
Atoms are made up of a nucleus, protons and electrons
what does fire need to burn
Answers
Answer:
Fuel and Oxygen
Explanation:
If 150 grams of water is to be heated from 15.0°C to 100°C to make a
cup of tea, how much heat must be added? The specific heat of water is
4.18 J/gºC *
Answers
Answer:
53295J
Explanation:
The amount of heat to be added can be calculated using the formula:
Q = m × c × ∆T
Where;
Q = amount of heat (J)
m = mass (g)
c = specific heat capacity of water
∆T = change in temperature (°C)
According to the provided information, m = 150g, c = 4.18 J/gºC, final temperature= 100°C, initial temperature= 15°C
Hence, Q = m × c × ∆T
Q = 150 × 4.18 × (100°C - 15°C)
Q = 627 × 85
Q = 53295J
The amount of heat added is 53295J
The smallest atomic unit which maintains the physical properties of a compound is a(n)
Answers
Answer:
The smallest atomic unit which maintains the physical properties of a compound is called a molecule.
Explanation:
A molecule is the smallest unit of a compound that can exist independently and still retain the properties of that compound. It is made up of two or more atoms that are bonded together, and it is the basic unit of a chemical compound. The atoms in a molecule are held together by chemical bonds, which are the attractive forces that hold the atoms together. Molecules can be made up of different types of atoms, and the type and arrangement of atoms in a molecule determine the properties of the compound.
For the following reaction, 35.0 grams of zinc oxide are allowed to react with 6.85 grams of water . zinc oxide (s) + water (I) ⟶ zinc hydroxide ( aq ) What is the maximum amount of zinc hydroxide that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete?
Answers
10.63 grams of ZnO remain after the reaction is complete.
The balanced chemical equation for the reaction between zinc oxide and water is:
ZnO(s) + H2O(l) → Zn(OH)2(aq)
No. of moles of ZnO = Mass of ZnO / Molar mass of ZnO= 35.0 g / 65.38 g/mol= 0.535 moles of ZnO
The amount of water is given as 6.85 g
The molar mass of water is:H2O = 18.02 g/mol
No. of moles of H2O = Mass of H2O / Molar mass of H2O= 6.85 g / 18.02 g/mol= 0.380 moles of H2O
Now, we need to find out the limiting reagent.
.No. of moles of Zn(OH)2 formed from 0.535 moles of ZnO = 0.535 molesNo. of moles of Zn(OH)2 formed from 0.380 moles of H2O = 0.380 moles
Therefore, since the amount of ZnO (0.535 moles) is greater than the amount of H2O (0.380 moles), H2O is the limiting reagent and ZnO is the excess reagent.
The maximum amount of Zn(OH)2 that can be formed is given by the amount of ZnO that reacts with H2O, which is 0.380 moles.
No. of grams of Zn(OH)2 = No. of moles of Zn(OH)2 × Molar mass of Zn(OH)2= 0.380 mol × (97.41 g/mol)= 37.08 gThe formula for the limiting reagent is H2O. The amount of excess reagent remaining after the reaction is complete can be calculated by subtracting the amount of limiting reagent used from the initial amount of excess reagent
.Initial amount of excess reagent (ZnO) = 35.0 g
No. of moles of ZnO = Mass of ZnO / Molar mass of ZnO= 35.0 g / 65.38 g/mol= 0.535 moles of ZnO
Amount of ZnO used in the reaction = No. of moles of Zn(OH)2 formed × Ratio of ZnO to Zn(OH)2= 0.380 mol × (1 mol ZnO / 1 mol Zn(OH)2)= 0.380 moles of ZnO used
Amount of ZnO remaining after the reaction = Initial amount of ZnO − Amount of ZnO used= 35.0 g − (0.380 mol × 65.38 g/mol)= 10.63 g
Therefore, 10.63 grams of ZnO remain after the reaction is complete.
learn more about zinc oxide here
https://brainly.com/question/30765370
#SPJ11
PLEASE HELP 20 POINTS

Answers
1. You can compare osmolarities of two solutions. Solution A=1Osm Glucose Solution B=2.5Osm Glucose Solution C=1OsmNaCl Answers: i) A is to B (hyperosmotic, isosmotic, hypoosmotic) ii) B is to A (hyperosmotic, isosmotic, hypoosmotic) iii) A is to C (hyperosmotic, isosmotic, hypoosmotic) iv) C is to A (hyperosmotic, isosmotic, hypoosmotic) 2. Body fluid osmolarity is 300mOsm. How will the following values change when you drink water? Would they increase, decrease, or not change?
Answers
i) A is to B: hypoosmotic (Solution A has a lower osmolarity compared to Solution B). ii) B is to A: hyperosmotic (Solution B has a higher osmolarity compared to Solution A)
iii) A is to C: isosmotic (Solution A and Solution C have the same osmolarity)
iv) C is to A: isosmotic (Solution C and Solution A have the same osmolarity)
When you drink water, the osmolarity of body fluids will decrease. This is because water is a hypotonic solution compared to body fluids.
Hypotonic refers to a solution that has a lower solute concentration compared to another solution or a reference solution. In a hypotonic solution, there is a higher concentration of water molecules relative to solute particles.
By drinking water, you are diluting the solute concentration in the body, leading to a decrease in osmolarity. Therefore, the values related to osmolarity, such as the concentration of solutes in the body fluids, would decrease.
To learn more about osmolarity
https://brainly.com/question/33463094
#SPJ11
Nucle acids are made up of smaller subunits called
Answers
Nucleic acids are made up of smaller subunits called nucleotides
Nucleotides, which are smaller components, make up nucleic acids. A monomer, also known as a building block, of nucleic acids like RNA and DNA is known as a nucleotide. A repeating pattern of the sugar-phosphate backbone with the nitrogenous bases spreading out as the rungs"of the DNA or RNA ladder is created.
The ladder is created when nucleotides are connected together by covalent bonds established between the phosphate group of one nucleotide and the sugar molecule of another nucleotide. This configuration of nucleotides creates the double helix structure in DNA and a number of secondary structures in RNA, which are essential for their biological roles as genetic information carriers and transmitters.
Read more about nucleotides on:
https://brainly.com/question/29392041
#SPJ4
What percentage of an original polonium sample will remain after 3 half-lives have passed?
Answers
After three half-lives, 12.5% of the original sample of polonium remains.
What is polonium element?Polonium is a very infrequent natural element. It is found in uranium ores but it is careless to extract it. It is obtained by bombarding bismuth-209 with neutrons to give bismuth-210, which then purifies to form polonium. All the industrially produced polonium in the world is made in Russia.Po-210 is an effect of the radioactive decay of uranium-238, which decays to radon-222 and then to polonium. Polonium 210 has a half-life of 138 days. Let the opening value of polonium-218 be 100. First half-life. Therefore, 6.25 % of the original sample remains after 4 half-lives that are 12 minutes. Element Polonium has atomic Number 84, p-block, Mass number is 209.
So we can conclude that polonium is a radioactive, silvery-gray, or black metallic element of the oxygen group in the periodic table.
Learn more about polonium here: https://brainly.com/question/16979893
#SPJ1
In this activity, you will use a gas properties simulation to analyze how the variables that describe a gas relate to each other under different conditions. In the center of the simulation is a box with a lid. The box can be filled with gas particles. There’s also a thermometer and a pressure gauge. You can choose the type of particle you want. You can also change the energy of the particles by heating or cooling the box.
The controls on the right panel allow you to hold certain parameters constant or to change the number of particles in the box. Near the bottom are options to pause and reset the simulation.

Answers

Need help!!!!!!!!!!!!!

Answers
Answer:
density
Explanation:
consider the following reaction. the dotted arrow is a placeholder. select the descriptor that best describes the relationship between the reactants and products.
Answers
The descriptor that best describes the relationship between the reactants and products is Decomposition.
Reactants are transformed into products during chemical reactions. The components that initiate a reaction are known as reactants, whereas the compounds that are produced as a result of that reaction are known as products.
CaO + CO2 = CaCO3
Calcium carbonate (CaCO3) breaks down into calcium oxide (CaO) and carbon dioxide (CO2) gas in this process.
The interaction between reactants and products in a chemical process can be described in a number of ways, including:
Conversion: The chemical process transforms the reactants into the products.
Synthesis: A single product is created when the reactants come together.
Decomposition: The breakdown of a single reactant into two or more products.
One or more reactants' components or functional groups are exchanged for another's, resulting in the formation of products.
Learn more about Decomposition
https://brainly.com/question/8009068
#SPJ4
Complete question
consider the following reaction.
CaO + CO2 = CaCO3
The dotted arrow is a placeholder. select the descriptor that best describes the relationship between the reactants and products.
In the heating and cooling curves below, identify the letter in the diagram diagram that corresponds to each of the listed processes in the table
I’m so confused if anyone could help (and explain as if I’m a 3 yr old) that would be helpful
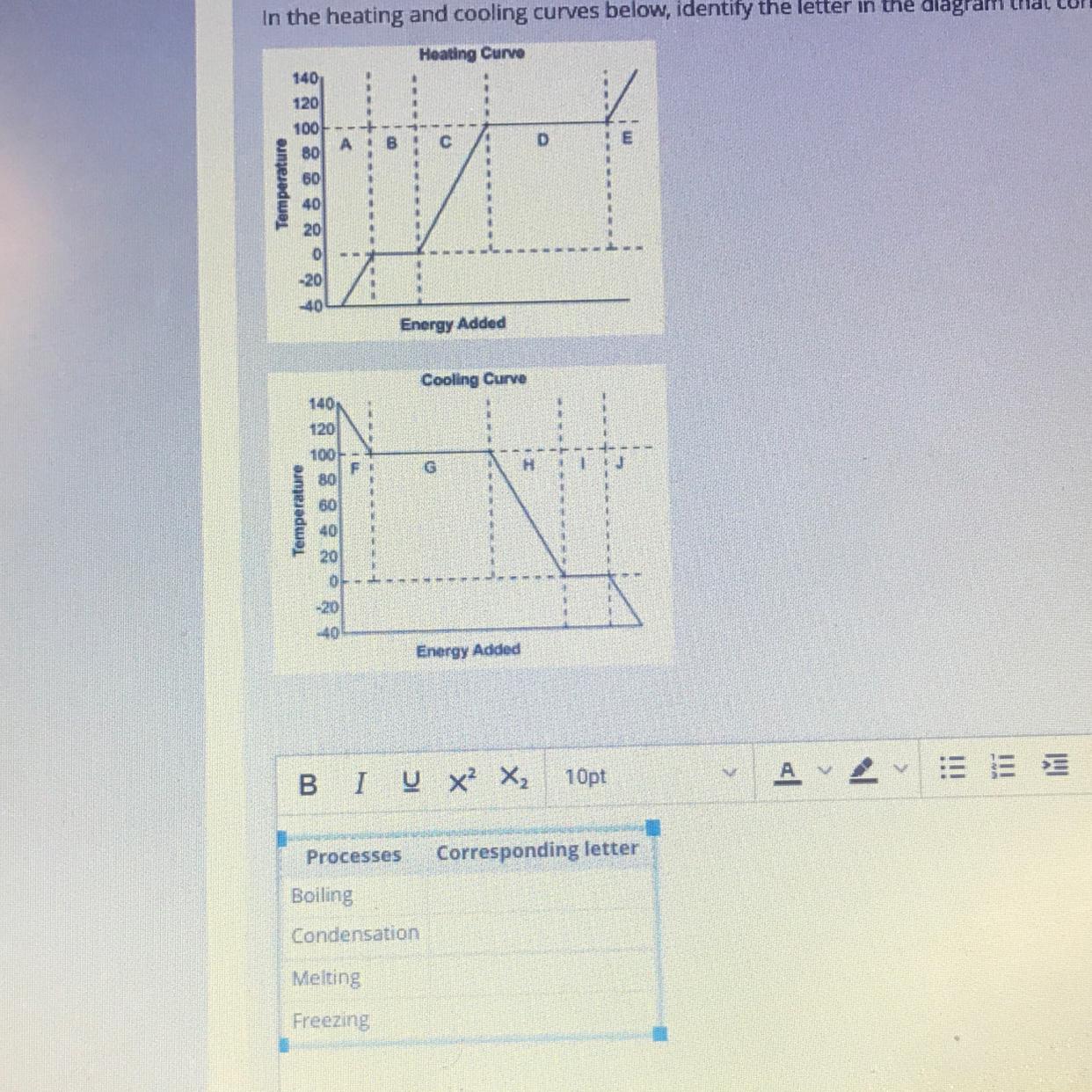
Answers
Answer:
Test for the first one is the best for
What is the empirical formula of a compound that consists of 39.99% carbon, 6.73% hydrogen, and 53.28% oxygen
Answers
Answer:
C3H6O4
Explanation:
What is the empirical formula of a compound that consists of 39.99% carbon, 6.73% hydrogen, and 53.28% oxygen
assume 1000 gm of the compound
C= 399.9 gm or 399.9/12.01 = 33.3 moles of C
H = 67.3 gm or 67.3/1.01 = 66.6 moles of H
O = 532.8 gm or 532.8/ 12.01 = 44.4 moles of O
the compound has a ratio of C to H to O of 3 to 6 to 4
so C3H6O4
Which of the following is not an alteration of a document?
additions
obliteration
erasure
O all of these answers
charring
None of these answers
ill give brainley
Answers
Given the following reaction at 1000 K and 1 bar: C₂H4(g) + H₂O(g) C2H5OH (g) Determine the equilibrium constant and its maximum conversion for an equimolar feed. Assume the standard enthalpy of reaction as a function of temperature. P4 P5 With reference to P4, now the reactor pressure is increased to 500 bar. What is the maximum possible conversion? Use the van der Waals equation and the Lewis fugacity rule to account for gas-phase nonideality.
Answers
The equilibrium constant and maximum conversion cannot be determined without additional information such as the standard enthalpy of reaction at 1000 K.
What is the relationship between pH and pOH in aqueous solutions?To determine the equilibrium constant and maximum conversion for the given reaction at 1000 K and 1 bar, you would need additional information such as the standard enthalpy of reaction at that temperature. Without that information, it's not possible to calculate the equilibrium constant or maximum conversion.
Regarding the reference to P4 and increasing the reactor pressure to 500 bar, the maximum possible conversion can be estimated by considering the effect of pressure on the equilibrium position. Increasing the pressure will shift the equilibrium towards the side with fewer moles of gas. Since the reaction involves a decrease in the number of moles of gas (2 moles of reactants to 1 mole of product), increasing the pressure will favor the formation of the products.
To calculate the maximum possible conversion, you would need to use equations that consider the non-ideality of gases, such as the van der Waals equation and the Lewis fugacity rule. These equations account for the deviations from ideal gas behavior due to intermolecular forces and molecular size. By incorporating these corrections, you can obtain more accurate results for the maximum conversion.
However, the specific calculations and equations involved in determining the maximum conversion using the van der Waals equation and the Lewis fugacity rule can be complex and require detailed knowledge of thermodynamics. It is recommended to consult your course materials or seek guidance from your instructor to understand and solve this problem accurately.
Learn more about standard enthalpy
brainly.com/question/28303513
#SPJ11
An air bubble with a volume of 5.0 ml is released at the bottom of a lake where the pressure is 3.0 atm. when it reaches the surface, the bubble experiences a pressure of 1.0 atm. formula to use: p1v1 = p2v2 how will the volume of the air bubble change?
Answers
The new volume of the air bubble that has an initial volume of 5.0 ml released at the bottom of a lake where the pressure is 3.0 atm is 15mL.
How to calculate volume?The volume of a given gas can be calculated by using the following formula:
P1V1 = P2V2
Where;
P1 = initial pressureV1 = initial volumeP2 = final pressureV2 = final volume5 × 3 = 1 × V2
15 = V2
V2 = 15mL
Therefore, the new volume of the air bubble that has an initial volume of 5.0 ml released at the bottom of a lake where the pressure is 3.0 atm is 15mL.
Learn more about volume at: https://brainly.com/question/1578538
#SPJ4
Answer: It will increase
Explanation:
How many moles are in 0.0688 g of silver chloride (AgCl)
Answers
Answer: 0.00048
m
o
l
Explanation:
Moles = Mass/Molar mass
n
=
m
M
r
=
0.0688
107.87
+
35.5
=
0.00048
m
o
l
after the technician adds 15.7115.71 ml of the kohkoh solution, the ph of the mixture is 4.094.09 . determine the pKapka of the weak acid.
Answers
The pKa value of a weak acid can be determined based on the pH of the mixture after adding a known volume of a strong base. In this case, the technician added 15.71 ml of KOH solution, resulting in a pH of 4.09.
To determine the pKa, we need to use the Henderson-Hasselbalch equation, which relates the pH, pKa, and the concentrations of the weak acid and its conjugate base: pH = pKa + log([conjugate base]/[weak acid]) We can rearrange this equation to solve for the pKa:
pKa = pH - log([conjugate base]/[weak acid])
However, we are missing the concentrations of the weak acid and its conjugate base. Without that information, we cannot calculate the pKa value accurately.
To know more about pKa visit:
brainly.com/question/32305719
#SPJ11
Which of the following is the correct definition of conduction?
A. the transmission of heat across empty space
B. the transition of heat across matter
C. the transfer of heat by currents within a liquid or gas
Answers
Answer:
B. The transition of heat across matter
Answer:
I Would THINK: B
because the definition is: "The process by which heat is transferred through a substance when there is a difference of temperature."
The density of a gas was found to be 19.1 g/L at 2.25 atm and 19.3°C. What is the molar mass of
the gas?
Answers
Answer:
Explanation:
Recall that the density of a gas is its mass to volume ratio,
ρ
=
m
V
. Therefore, if we can determine the mass of some volume of a gas, we will get its density. The density of an unknown gas can used to determine its molar mass and thereby assist in its identification. The ideal gas law, PV = nRT, provides us with a means of deriving such a mathematical formula to relate the density of a gas to its volume
Which parts must be balanced in a chemical equation? (1
O energy on the reactant and product sides
O numbers of reactant and product molecules
O states of reactants and products
O
numbers of reactant and product atoms
Answers
In a chemical equation the numbers of reactant and product atoms needs to balanced.
Balancing To keep a chemical equation balanced, there should be an equal number of each type of atom on both sides of the equation. A coefficient is a numerical value that precedes a chemical symbol or formula. This indicates that a number of atoms or molecules of matter are involved in the reaction.According to the law of conservation of mass, in a chemical reaction the mass of the product must equal the mass of the reactants. Therefore, the amount of atoms of each element does not change in chemical reactions. Therefore, the chemical equations representing chemical reactions must be balanced. A balanced chemical equation occurs when the number of atoms involved on the reactant side is equal to the number of atoms on the product side.For more information on balanced chemical equation kindly visit to
https://brainly.com/question/28294176
#SPJ1
if .296 j of heat causes a .661 degree c temperature change, what mass of water is present?
Answers
Answer:
m=0.000107 kg
Explanation:
H=m*cp*change in temperature
0.296=m*4182*0.661
m=0.000107 kg