For the reaction A(aq) + B(aq) <---> C(aq) + D(aq), the equilibrium constant is 20. 4 at 25oC and 37. 1 at 50oC. What is the value of the standard Gibbs free energy change (in kJ) of this reaction at 75oC?
Answers
The value of the standard Gibbs free energy change (ΔG°) for the reaction at 75°C is approximately -2.457 kJ/mol.
To determine the value of the standard Gibbs free energy change (ΔG°) for the reaction A(aq) + B(aq) ↔ C(aq) + D(aq) at 75°C, we can use the Van 't Hoff equation, which relates the equilibrium constant (K) to the temperature (T) and ΔG°:
ln(K2/K1) = -ΔH°/R * (1/T2 - 1/T1)
Given:
Equilibrium constant at 25°C (K1) = 20.4
Equilibrium constant at 50°C (K2) = 37.1
Temperature at 25°C (T1) = 298 K
Temperature at 50°C (T2) = 323 K
R = 8.314 J/(mol·K) (gas constant)
We need to solve for ΔH°, the enthalpy change at standard conditions. Rearranging the equation:
ΔH° = -R * (ln(K2/K1) / (1/T2 - 1/T1))
Calculating:
ΔH° ≈ -8.314 J/(mol·K) * (ln(37.1/20.4) / (1/323 K - 1/298 K))
Now, we can use the relationship between ΔG° and ΔH° at constant temperature:
ΔG° = ΔH° - TΔS°
At constant temperature, ΔG° = ΔH° - TΔS°
Given that the reaction is at standard conditions, ΔS° is zero (since it is not provided). Thus, ΔG° = ΔH°.
Converting J to kJ:
ΔG° ≈ ΔH° / 1000 kJ
Now, we can substitute the calculated value of ΔH° into the equation to find ΔG°:
ΔG° ≈ -8.314 J/(mol·K) * (ln(37.1/20.4) / (1/323 K - 1/298 K)) / 1000 kJ
Calculating:
ΔG° ≈ -2.457 kJ/mol
Therefore, the value of the standard Gibbs free energy change (ΔG°) for the reaction at 75°C is approximately -2.457 kJ/mol.
learn more about standard Gibbs here
https://brainly.com/question/30654218
#SPJ11
Related Questions
Plz help I need the answer now!! Which statement is best supported by the evidence on the map?
A. Oil is produced mostly in the southern part of Africa.
B. Gold is found in every country in Africa.
O
C. Iron ore is found mostly in northern Africa.
D. Each country in Africa has about the same amount of each natural
resource.

Answers
Answer:
I'd say C. You can eliminate the rest of the answers and get C. Most of the iron is found up North on the map.
Explanation:
a block of copper of unknown mass has an initial temperature of 65.4 ∘c . the copper is immersed in a beaker containing 95.7 g of water at 22.7 ∘c . when the two substances reach thermal equilibrium, the final temperature is 24.2 ∘c . what is the mass of the copper block?
Answers
To determine the mass of the copper block, we can use the principle of conservation of energy. The heat lost by the copper block will be equal to the heat gained by the water in the beaker.
The equation for heat transfer is Q = m * c * ΔT, Where: Q is the heat transferred, m is the mass, c is the specific heat capacity and ΔT is the change in temperature.
The specific heat capacity of copper is approximately 0.39 J/g·°C, and for water, it is about 4.18 J/g·°C.
Let's calculate the heat gained by the water:
Q_water = m_water * c_water * ΔT_water
m_water = 95.7 g (mass of water)
c_water = 4.18 J/g·°C (specific heat capacity of water)
ΔT_water = (final temperature - initial temperature) = (24.2 °C - 22.7 °C) = 1.5 °C
Q_water = 95.7 g * 4.18 J/g·°C * 1.5 °C = 599.595 J
Now, let's calculate the heat lost by the copper block:
Q_copper = m_copper * c_copper * ΔT_copper
c_copper = 0.39 J/g·°C (specific heat capacity of copper)
ΔT_copper = (final temperature - initial temperature) = (24.2 °C - 65.4 °C) = -41.2 °C
We have ΔT_copper as a negative value because the copper block loses heat.
Q_copper = m_copper * 0.39 J/g·°C * (-41.2 °C) = -16.068 m_copper J
According to the principle of conservation of energy, the heat gained by the water is equal to the heat lost by the copper block:
Q_water = Q_copper
599.595 J = -16.068 m_copper J
Solving for m_copper:
m_copper = 599.595 J / (-16.068 J/g)
m_copper ≈ -37.41 g
Learn more about principle of conservation of energy here ;
https://brainly.com/question/16881881
#SPJ11
To find the mass of the copper block, we can use the equation for heat transfer. The heat lost by the copper block is equal to the heat gained by the water.
Explanation:To determine the mass of the copper block, we can use the principle of heat transfer, specifically the equation for heat gained or lost. In this case, the heat lost by the copper block is equal to the heat gained by the water.
We can use the equation: heat lost by copper = heat gained by water.
Plugging in the given values, we can solve for the mass of the copper block.
Learn more about Heat transfer here:https://brainly.com/question/34419089
#SPJ11
what current is required to produce 91.6 g of chromium metal from chromium(vi) oxide in 12.4 hours?
Answers
A current of 2.41 A is required to produce 91.6 g of chromium metal from chromium(VI) oxide in 12.4 hours.
The calculation to determine the required current to produce a certain amount of chromium metal from chromium(VI) oxide involves using Faraday's law, which relates the amount of substance produced to the current and time. The molar mass of Cr2O3 is 151.99 g/mol, and the reduction of Cr2O3 to Cr involves the transfer of six electrons per mole.
Using these values and Faraday's constant (96,485 C/mol e-), we can calculate the required current as follows:
Calculate the number of moles of Cr2O3:
91.6 g / 151.99 g/mol = 0.603 mol
Calculate the amount of charge needed to reduce the Cr2O3:
6 mol e- / mol of Cr2O3 x 0.603 mol x 96,485 C/mol e- = 350,409 C
Calculate the current required:
350,409 C / (12.4 h x 3600 s/h) = 2.41 A
Therefore, a current of 2.41 A is required to produce 91.6 g of chromium metal from chromium(VI) oxide in 12.4 hours.
To know more about chromium metal, refer here:
https://brainly.com/question/30722265#
#SPJ11
why are so many products made from plastic ?
Answers
Explanation:
Plastic takes time to degrade which means it has great longevity. Plastic does not break as easily as glass or other materials. It lasts long and offers great service. Plastic storage containers offer greater flexibility than any other packaging materials.
what are three ways of measuring the amount of substance?
Answers
Answer:
Measure its mass
Measure its volume
Measure its number of moles
Explanation:
When a student adds 30.0 mL of 1.00 M HCl to 0.56 g of powdered Fe, a reaction occurs according to the equation above. When the reaction is complete at 273 K and 1.0 atm, which of the following is true?
Answers
The true statement is "HCl is in excess, and 0.100 mol of HCl remains unreacted" Therefore, option A is correct.
Given:
Molar mass of Fe = 55.85 g/mol
Mass of Fe = 0.56 g
Moles of Fe = Mass of Fe / Molar mass of Fe
Moles of Fe = 0.56 g / 55.85 g/mol
Moles of Fe ≈ 0.01 mol
According to the balanced equation, the mole ratio between Fe and HCl is 1:2. This means that for every 1 mole of Fe, 2 moles of HCl are required.
Therefore, the moles of HCl required to react completely with the given amount of Fe are:
Moles of HCl = 2 × Moles of Fe
Moles of HCl = 2 × 0.01 mol
Moles of HCl = 0.02 mol
Volume of HCl solution = 30.0 mL = 30.0 mL × (1 L / 1000 mL) = 0.0300 L
Molarity of HCl = 1.00 M
Moles of HCl = Molarity × Volume
Moles of HCl = 1.00 M × 0.0300 L
Moles of HCl = 0.0300 mol
Since the moles of HCl required to react completely with Fe are 0.02 mol and the initial moles of HCl present are 0.0300 mol, we can see that HCl is in excess.
To learn more about mol, follow the link:
https://brainly.com/question/31329389
#SPJ12
Your uestion is incomplete,complete question is:
Fe(s) + 2HCl(aq) ⇒ FeCl₂(aq) + H2(g)
When a student adds 30.0 mL of 1.00 M HCl to 0.56 g of powdered Fe, a reaction occurs according to the equation above. When the reaction is complete at 273 K and 1.0 atm, which of the following is true?
A) HCl is in excess, and 0.100 mol of HCl remains unreacted.
D) 0.22 L of H2 has been produced.
The correct answer is D. I can't figure out why A is wrong.
A net force of 16 N causes a mass to accelerate at a rate of 5 m/s2.
Determine the mass in kg.
Answers
Answer:
3.2 kg
Explanation:
f=ma
16=m*5
16/5=m
3.2
write four facts of cross breeding plants
Answers
Answer:
Plant breeding
Plant breeding is the science of changing the traits of plants in order to produce desired characteristics. It has been used to improve the quality of nutrition in products for humans and animals.
Explanation:
Which of the following means a natural occurring solid that has a specific chemical makeup?
A. Rock
B. Mineral
C. Erosion
Answers
Answer:
mineral
Explanation:
Because every natural thing has mineral
What factors determine the amount of energy stored in a gummy bear, and how do they affect the amount of energy stored?
Some ideas to get you started: Type of bond, number of bonds, etc
Answers
Answer:i don’t know
Explanation:i don’t know
How much anhydrous magnesium sulfate is required to dry a diethyl ether solution after preforming extractions involving water?.
Answers
The anhydrous magnesium sulfate required to dry a di-ethyl ether solution after performing extractions involving water is enough such that some powder remains free flowing in solution with swirling without clumping.
Extraction is a process which involves transfer of a solute from one phase to another. A cup of tea or coffee prepared represents a process of extraction.The flavor and odor components are extracted from the dried material into the water.
Anhydrous magnesium sulphate works as a drying agent. Magnesium sulfate (MgSO₄) works by forming complex with H₂O in the solvent. It forms a hydrated MgSO₄ precipitate. This precipitate is then filtered out by gravity, which yields an anhydrous product. Due to the high affinity towards water molecules, MgSO₄ removes any amount of water from the extracts.
Due to the presence of an active hydrogen atom, ethyl alcohol reacts with magnesium metal. Diethyl ether, does not have any replaceable hydrogen atom. So, it does not react with magnesium sulfate, therefore, can be dried by anhydrous magnesium sulfate.
Complete question is -
How much anhydrous magnesium sulfate is required to dry a diethyl ether solution after preforming extractions involving water?
Select answer and submit.
a. Enough to cover the tip of your scoopula once
b. 1 g/mL of diethyl ether
c. Enough such that some powder remains free flowing in solution with
swirling without clumping
d. Anhydrous magnesium sulfate doesn't dry diethyl ether
To learn more about extraction,
brainly.com/question/14038722
#SPJ1
how do we know interstellar amtter is hydrogen and helium
Answers
The interstellar matter is the hydrogen and the helium will gives the narrow absorption lines in the spectra of the some stars.
The interstellar matter is composed of the multiple phases that will distinguished by the whether matter is the ionic, atomic, or the molecular, and the temperature and the density of the matter. The interstellar medium is the composed the primarily, of the hydrogen atom , followed by the helium atom with the trace amounts of the carbon, the oxygen, and the nitrogen.
The matter will creates the narrow absorption of the lines in the spectra of the some of the stars.
To learn more about interstellar here
https://brainly.com/question/13034266
#SPJ4
here are four sketches of substances. each sketch is drawn as if a sample of the substance were under a microscope so powerful that individual atoms could be seen. decide whether each sketch shows a sample of an element, a compound, or a mixture.
Answers
Substance X - compound because it is a combination of elements
Substance Y - Mixture because it is a combination of substances
Substances Z - Element because it contains atoms
Substance T - compound because it is a combination of elements
What are the samples?We know that an atom is the smallest particle of a substance that can take part in a chemical reaction. A compound is formed by a mixture of atoms. An element is obtained as the smallest independent part of a substance.
Now let use classify the samples shown in the images attached.
Substance X - compound because it is a combination of elements
Substance Y - Mixture because it is a combination of substances
Substances Z - Element because it contains atoms
Substance T - compound because it is a combination of elements
Learn more about compounds:https://brainly.com/question/13516179?
#SPJ1

How many hours are in 25 days??
Answers
explanation
each day has 24 hours and we want to know how much hours is equivalent to 25 days so we multiply 24 times 25 which is 600.
How to find moles of a cooking recipe? Chemistry project help please

Answers
The moles of substances required to make scrambled eggs is given in the cooking recipe for scrambled eggs.
What is the moles of a substance?A mole of. a substance is the amount of that substance which contains the avogadro number (6.02 × 10^23) of particles in it.
A mole of a substance is usually given as a standard unit measurement of that substance.
The mass of 1 mole of is known as the molar mass if that substance.
From the recipe for preparing scramble eggs given, the moles of substances required are as follows:
6 moles of eggs1 mole of red bell pepper1 mole of green bell pepper1/2 moles of carrots1/4 moles of olive oil 1/4 cup of saltTherefore, the moles of substances required to make scrambled eggs is given in the cooking recipe for scrambled eggs.
Learn more about moles of substances at: https://brainly.in/question/132101
#SPJ1
a farmer notices that the nitrates (no3) from his fertilizer are disappearing rapidly from his soil. this could be due to:
Answers
The farmer noticed that the nitrates (NO3) from his fertilizer are disappearing rapidly from his soil. This could be due to several reasons, including: Leaching, Denitrification, Plant Uptake.
Leaching: This is the process whereby nitrates are washed away from the soil by rainfall or irrigation. When there is heavy rainfall or excessive watering, nitrates can be washed away from the topsoil, leaving the plants without the required nutrients.
Denitrification: This is a process whereby bacteria in the soil break down nitrates into nitrogen gas, which is released into the atmosphere. This process can occur in poorly drained soil, which is waterlogged and lacks sufficient oxygen to support plant growth.
Plant Uptake: Nitrogen is a vital nutrient for plant growth, and plants require it to develop leaves, stems, and roots. When plants absorb the nitrogen from the soil, the nitrates in the soil reduce significantly.In conclusion, several factors could lead to the rapid disappearance of nitrates from the soil. The farmer needs to understand the primary cause of the problem to address it effectively. Leaching, denitrification, and plant uptake are some of the reasons the nitrates could be disappearing rapidly from the soil.
for more such question on fertilizer
https://brainly.com/question/24782241
#SPJ11
Which sphingolipids have a single monosaccharide as a component? A. cerebrosides B. gangliosides C. globosides D. plasmalogens
Answers
The sphingolipids that have a single monosaccharide as a component are cerebrosides. Option A is correct.
Cerebrosides are a type of sphingolipid, which are a class of lipids found in cell membranes. They are composed of a sphingosine backbone, a fatty acid chain, and a single monosaccharide unit. The monosaccharide component is typically glucose or galactose.
The structure of cerebrosides consists of a long-chain amino alcohol called sphingosine, which is connected to a fatty acid via an amide bond. The fatty acid chain is hydrophobic and anchors the cerebroside within the lipid bilayer of cell membranes. The monosaccharide is attached to the sphingosine backbone through a glycosidic linkage.
Cerebrosides are particularly abundant in the myelin sheath, a protective covering around nerve fibers in the nervous system. They contribute to the structural integrity and insulation of nerve fibers, allowing for efficient transmission of nerve impulses.
On the other hand, gangliosides, globosides, and plasmalogens are sphingolipids that contain more complex carbohydrate structures with multiple monosaccharides or additional components.
Hence, A. is the correct option.
To know more about sphingolipids here
https://brainly.com/question/31662027
#SPJ4
Cerebrosides are the sphingolipids that have a single monosaccharide as a component.
sphingolipids are a class of lipids that contain a sphingoid base as their backbone. They are important components of cell membranes and play various roles in cell signaling and recognition. Sphingolipids can be classified into different subgroups based on their structure and composition.
One such classification is based on the presence of monosaccharides as components. cerebrosides, gangliosides, globosides, and plasmalogens are all types of sphingolipids.
Cerebrosides have a single monosaccharide as a component. They are composed of a sphingosine backbone linked to a single monosaccharide molecule. Cerebrosides are found in various tissues and play important roles in cell signaling and cell adhesion.
Gangliosides and globosides, on the other hand, have multiple monosaccharides as components. Gangliosides contain one or more sialic acid residues, while globosides contain multiple neutral monosaccharides.
Plasmalogens, another type of sphingolipid, do not contain monosaccharides as components. They are characterized by the presence of a vinyl ether bond at the sn-1 position of the glycerol backbone.
Learn more:About sphingolipids here:
https://brainly.com/question/31662027
#SPJ11
flowback waste water is disposed of in a process called deep well injection which plumps large quantities of waste water down into porous sandstone and limestone rock formations underground. what potential problems could result from this?
Answers
The potential problems associated with a deep well injection is that it can result in polluting underground water.
If there are many wells nearby, injecting wastewater into subsurface rock strata can be problematic. Consider porous sandstone, which contains minute openings. Water under high pressure, such as wastewater from fracking, can penetrate the sandstone and travel with underground water.
An injection well is employed to inject fluid underground into porous geologic formations. These subterranean structures might be anything from a modest soil layer to thick sandstone or limestone. Water, wastewater, brine (salt water), and water that has been combined with chemicals are all examples of injected fluids.
To know more about an injection well, refer to the following link:
https://brainly.com/question/4472460
#SPJ1
How many moles are in 3. 01 x 10^22 atoms of magnesium.
Answers
there are 0.05 moles
A 200g piece of iron absorbs 8980 joules to raise the temperature from 20°C to 120°C. Find the specific heat of iron?
Answers
Answer:
The specific heat of iron is 0.449 J/g°C.
Explanation:
First, let's see the formula of specific heat:
\(c=\frac{q}{m\cdot\Delta T}\begin{cases}{q=amount\text{ of heat \lparen Joules\rparen}} \\ {m\text{ = mass of substance \lparen grams\rparen}} \\ \Delta T={Change\text{ of temperature \lparen\degree C\rparen}}\end{cases},\)Now, we have to replace the given data in the formula, where q = 8980 J, m = 200 g and ΔT = Final temperature - Initial temperature = 120 °C - 20 °C = 100 °C:
\(c=\frac{8980\text{ J}}{200\text{ g}\cdot100\degree C}=0.449\frac{J}{g\degree C}.\)The specific heat of iron is 0.449 J/g°C.
4. Why is it important to neutralize an acid spill
before attempting to clean it up?
Answers
Answer:
It the acid easier to handel
How does the government control scientific research
Answers
Answer:
The government allocates a budget for research every year. The spending of that money is determined by government priorities. Some of the money is spent directly, in government-funded research centers.
Other money is distributed to other research institutions.
Money spent by other institutions for research has no government oversight.
Explanation:
br2(g)+cl2(g)⇌2brcl(g) δg∘f for brcl(g) is -1.0 kj/mol
Answers
The rate constant of the reaction \(Br_{2}(g) + Cl_{2}(g) ------ > 2BrCl(g)\)is given as 1.002.
What is the rate constant?We know that the rate of reaction can be defined as how fast or slow that a reaction occurs. The rate of reaction can be obtained the free energy of the formation of the bromine chloride.
We know that;
Free energy of the reaction = Sum of free energy of the products - Sum of free energy of the products
Thus it is clear that;
ΔG reaction = 2(-1.0) - [(3.1) + 0]
ΔG reaction = -2 - 3.1
= -5.1 kJ/mol
Then using
ΔG = -RTlnK
R = gas constant
T = temperature
K = rate constant
-5.1 = -(8.314 * 298) lnK
lnK = -5.1 /-(8.314 * 298)
ln K = 2.1 * 10^-3
K = e^2.1 * 10^-3
K = 1.002
The reaction would be observed from the calculation to have a rate constant of 1.002.
Learn more about rate constant:https://brainly.com/question/14977272
#SPJ1
Missing parts;
Use data from Appendix IIB to calculate the equilibrium constants at 25 ∘C for each of the following reactions Br2(g)+Cl2(g)⇌2BrCl(g) ΔG∘f for BrCl(g) is -1.0 kJ/mol, Cl2 is 0, Br2 is 3.1
Please answer question #4
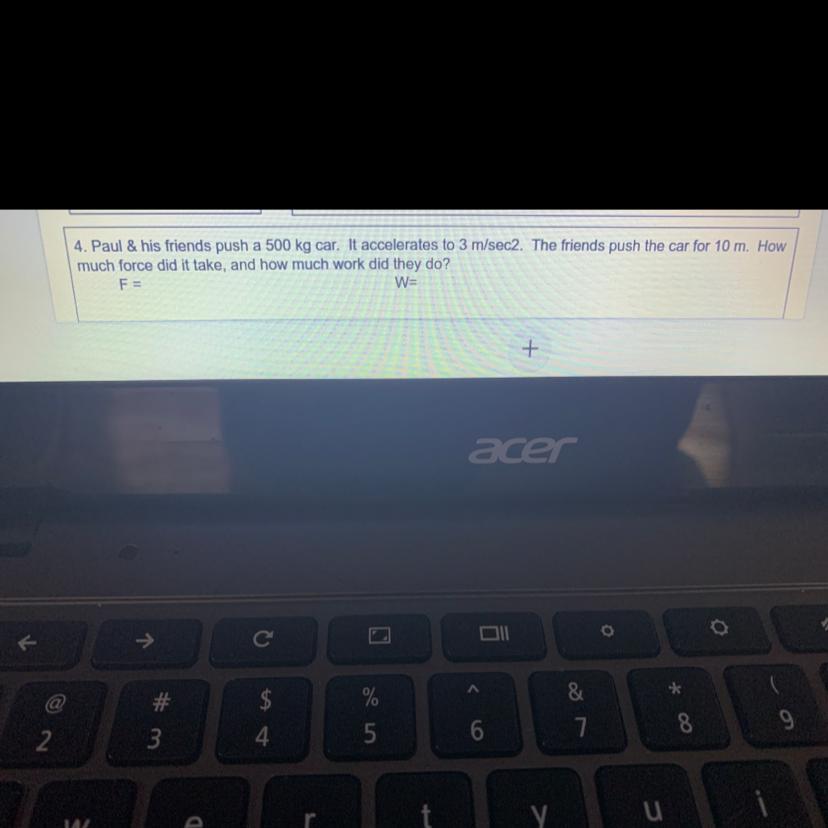
Answers
Answer:
F=500×3 = 1500 N
W = 1500×10 =15000 Nm
How fast can a turbidity bed form
Answers
Answer:
a matter of hours
Explanation:
Salt is both strong and brittle. These properties relate to its atomic bonds. Examine a toothpick and try to bend it. Then imagine hitting the end of an upright toothpick with a hammer. Is a toothpick a good model for showing the strength and brittleness of salt? Explain your answer.
Answers
Answer:
Yes, A toothpick can be a good model for showing the strength and brittleness of salt as It will break and splinter just like salt.
What are the properties of salt ?
Salts are ionic in nature due to the presence of ions.
They are brittle, hard and crystalline solids.
Salt is white, odorless and it has a salty taste.
Toothpick will break and splinter just like salt, exclamation, because it breaks easy and if we use a hammer on a tooth pick its gonna break and splinter just like salt.
Therefore , A toothpick can be a good model for showing the strength and brittleness of salt as It will break and splinter just like salt.
Explanation: hope this helps
In nature, All the atoms are?
Answers
Answer:
Made up of proton, electron, neutron and 40 different subatomic particles
1) For the precipitation reaction of calcium oxalate below, the Ks = 3.7x10 Note: For this question, do not apply the small x approximation. A) If excess calcium oxalate were added to 100.0 mL of pure water, what concentration of calcium ions and oxalate ions would be expected when the solution is saturated? B) If 1.00 mg of calcium chloride were then added to the mixture (assume no solution volume change and complete dissolution and dissociation of CaCl2), what would be the expected concentrations of calcium ions and oxalate ions once equilibrium is reestablished? Ca2+(aq) + C2042(aq) ⇄ CaC204(s)
Answers
A) In precipitation reaction when the solution is saturated, the expected concentration of both calcium ions and oxalate ions would be approximately 0.0192 mol/L.
B) After equilibrium is reestablished, the expected concentrations of calcium ions and oxalate ions are approximately 2.498 × 10⁻⁴ mol/L and 0.0192 mol/L, respectively.
To answer the given questions about the precipitation reaction of calcium oxalate, let's break it down into two parts:
A) The concentration of calcium ions and oxalate ions in the saturated solution can be determined when an excess amount of calcium oxalate is added to 100.0 mL of pure water.
Since excess calcium oxalate is added, it means that the solution will contain more calcium oxalate than what can dissolve. At saturation, the solution is in equilibrium with the solid calcium oxalate.
Let's assume the concentration of calcium ions and oxalate ions in the saturated solution is represented by "x" (in mol/L).
The equilibrium expression for the reaction is:
Ks = [Ca²⁺][C₂O₄²⁻]
Given that the equilibrium constant Ks = 3.7 × 10⁻⁴, we can set up the equation:
3.7 × 10⁻⁴ = x * x
Solving for "x," we find:
x = √(3.7 × 10⁻⁴) ≈ 0.0192 mol/L
Therefore, when the solution is saturated, the expected concentration of both calcium ions and oxalate ions would be approximately 0.0192 mol/L.
B) If 1.00 mg of calcium chloride (CaCl2) were added to the mixture, what would be the expected concentrations of calcium ions and oxalate ions once equilibrium is reestablished?
Since calcium chloride (CaCl₂) dissociates completely into calcium ions (Ca²⁺) and chloride ions (Cl⁻) in solution, the addition of 1.00 mg of CaCl₂ will result in the addition of 1.00 mg of calcium ions.
First, we need to convert the mass of calcium ions from mg to mol:
1.00 mg = 0.001 g
0.001 g / (molar mass of Ca²⁺) = 0.001 g / 40.08 g/mol ≈ 2.498 × 10⁻⁵ mol
Since the solution volume is assumed to be unchanged, the concentrations of calcium ions and oxalate ions will change but not the molar amounts.
The concentration of calcium ions is the molar amount (2.498 × 10^(-5) mol) divided by the total solution volume (100.0 mL or 0.100 L):
Concentration of calcium ions = (2.498 × 10⁻⁵ mol) / 0.100 L ≈ 2.498 × 10⁻⁴ mol/L
The concentration of oxalate ions remains the same as in part A since the addition of calcium chloride does not affect the concentration of oxalate ions.
Therefore, after equilibrium is reestablished, the expected concentrations of calcium ions and oxalate ions are approximately 2.498 × 10⁻⁴ mol/L and 0.0192 mol/L, respectively.
Learn more about precipitation reaction at: https://brainly.com/question/13016165
#SPJ11
Potassium sulfate is added to water. The equation that best represents this process
is:
K₂S(s) → K(s) + 2 S(s)
K₂S(s) K(s) + 2 S(s)
K₂SO4(s) → 2 K (aq) + SO4² (aq)
K₂S(s) 2 K (aq) + S² (aq)
K₂SO4(s) 2 K(s) + SO4(g)
K₂S(s)→2 K (aq) + S² (aq)
K₂SO4(s)→2 K(s) - SO4(g)
K₂SO4(s) 2 K (aq) + SO4²(aq)
Answers
The equation that best represents the addition of potassium sulfate to water is \(K_2SO_4(s) -- > 2 K^+ (aq) + SO4^{2-} (aq)\)
Dissolution of potassium sulfate in waterWhen potassium sulfate is dissolved in water, it dissociates into its cation and anion according to the following equation:
\(K_2SO_4(s) -- > 2 K^+ (aq) + SO4^{2-} (aq)\)
The positive ions are 2 moles of potassium ions while the negative ion is a mole of sulfate ion.
More on potassium sulfate can be found here: https://brainly.com/question/15405465
#SPJ1
Why do plants and animals adapt to their environment?
НЕ
А
to become better at getting food
B
to protect themselves against other animals
с
to become better able to survive in their surroundings
D
all of the above
Answers
Answer: all of the above
Explanation:
Plants and animals must be able to adapt to their surroundings, because otherwise they could be in danger because of a simple change weather patterns or different predators roaming around. They will also have possible competition.