how many dots would appear on the lewis electron dot diagram for an atom whose electron notation ends in 6s25d106p4?
Answers
An atom with an electron notation of 6s25d106p4 would have 6 dots on the Lewis electron dot diagram.
Are Lewis and electron dots the same?Lewis dot structures, also known as electron dot structures, are schematics that show the interatomic chemical bonds in a molecule. Additionally, they show how many lone pairs there are overall in each of the atoms that make up the molecule.
How is the electron dot sign read?Each symbol stands for the atom's nucleus and central electrons. Each "H" in this diagram stands for the nucleus of an atom of hydrogen, and each "O" for the nucleus and two core electrons of an atom of oxygen. The dots signify valence electrons that are not in bonds.
To know more about Lewis electron dot visit:
https://brainly.com/question/19093827
#SPJ4
Related Questions
A 996.9 g sample of ethanol undergoes a temperature change of -70.98 °C while releasing 62.9
calories of heat. What is the specific heat capacity of ethanol?
Answers
Answer:
\(c=3.71\ J/g^{\circ} C\)
Explanation:
Given that,
Mass of sample, m = 996.9 g
The change in temperature of the sample, \(\Delta T=-70.98^{\circ}C\)
Heat produced, Q = 62.9 calories = 263173.6 J
The heat released by a sample due to change in temperature is given by :
\(Q=mc\Delta T\)
Where
c is the specific heat capacity
So,
\(c=\dfrac{Q}{m\Delta T}\\\\c=\dfrac{263173.6}{996.9\times 70.98}\\\\c=3.71\ J/g^{\circ} C\)
So, the specific heat of ethanol is equal to \(3.71\ J/g^{\circ} C\).
Complete the balanced dissociation equation for the compound below in aqueous solution. If the compound does not dissociate, write NR after the reaction arrow. Be sure to include the proper phases for all species within the reaction. Mg(OH)2( s)→
Answers
The balanced dissociation equation for Mg(OH)₂ in aqueous solution is: Mg(OH)₂(s) → Mg²⁺(aq) + 2OH⁻(aq)
Mg(OH)₂ is a compound that dissociates in water to form magnesium ions (Mg²⁺) and hydroxide ions (OH⁻). In the solid state, Mg(OH)₂ exists as an undissociated compound. However, when it is dissolved in water, it dissociates into its constituent ions.
The balanced dissociation equation represents the separation of Mg(OH)₂ into its ionic components. The equation shows that one molecule of Mg(OH)₂ dissociates to form one Mg²⁺ ion and two OH⁻ ions. The charges on the ions are balanced to ensure overall charge neutrality.
The state of each species is indicated in the equation. Mg(OH)₂(s) represents the solid state of the compound, while Mg²⁺(aq) and OH⁻(aq) indicate that the magnesium and hydroxide ions are in the aqueous (dissolved) state.
learn more about balanced dissociation equation here:
https://brainly.com/question/14607214
#SPJ11
ANSWER THE QUESTION TO GET BRAINLIST
When sunlight travels from the vacuum of space into Earth's atmosphere it slowed down slightly. Explain why and name the light principle involved.
Answers
It is thought that global dimming is probably due to the increased presence of aerosol particles in Earth's atmosphere, caused by pollution, dust, or volcanic eruptions. Aerosols and other particulates absorb solar energy and reflect sunlight back into space. ... Water droplets in clouds coalesce around the particles.
Global Dimming
source:
wikipedia via gooogle snippet
PLS HELP I WILL GIVE BRAINLISTED AND I GIVE YOU SO MUCH STUFF FI NEED IT PLS

Answers
Photosynthesis removes CO2 and animal activities, burning fossil fuels and burning wood add CO2.
Why is CO2 needed?
The main greenhouse gas that helps keep our atmosphere warm is carbon dioxide. Without them, our earth would be uninhabitable. However, the rise in average global temperature due to rising levels of CO2 in our atmosphere is affecting other aspects of Earth's climate. Carbon dioxide is required for the internal respiration of the human body. Internal respiration is the process of removing carbon dioxide and delivering oxygen to body tissues.
The pH value of the blood is protected by the vital carbon dioxide. Therefore, only plants excrete CO2.
To learn more about Carbon dioxide, here
brainly.com/question/3049557
#SPJ1
Determine the number of moles of hydrogen atoms in each sample.
a. 0.0885 mol C4H10
b. 1.3 mol CH4
c. 2.4 mol C6H12
d. 1.87 mol C8H12
Answers
Answer: D
Explanation:
Determine the number of moles of hydrogen atoms in .0885 mol C4H10
I'm a bit stupid, that's why I'm going to ask for help, can you be nice and help me? Thank you

Answers
2. Valence electron
3. Isotopes
4.Covalent
5. ionic
Explanation:
ion
valence electrons
isotope
covalence
ionic
Consider the reaction N 2
( g)+3H 2
( g)⟶2NH 3
( g) Using the standard thermodynamic data in the tables linked above, calculate ΔG rxn
for this reaction at 298.15 K if the pressure of each gas is 12.52 mmHg. Consider the reaction 4HCl(g)+O 2
( g)⟶2H 2
O(g)+2Cl 2
( g) Using the standard thermodynamic data in the tables linked above, calculate ΔG for this reaction at 298.15 K if the pressure of each gas is 16.47 mmHg. ANSWER: k]/mol
Answers
To calculate the standard Gibbs free energy change (ΔG°) for a reaction using thermodynamic data, we can utilize the equation:
ΔG° = ΣnΔG°f(products) - ΣnΔG°f(reactants)
where ΔG°f is the standard Gibbs free energy of formation for each species and n represents the stoichiometric coefficients of each species in the balanced equation.
Reaction: N2(g) + 3H2(g) ⟶ 2NH3(g)
Partial pressure of N2(g) = 12.52 mmHg / 760 mmHg/atm = 0.016447 atm
Partial pressure of H2(g) = 12.52 mmHg / 760 mmHg/atm = 0.016447 atm
Partial pressure of NH3(g) = 12.52 mmHg / 760 mmHg/atm = 0.016447 atm
ΔG° = (2 * ΔG°f(NH3)) - (1 * ΔG°f(N2)) - (3 * ΔG°f(H2))
ΔG° = (2 * (-16.45 kJ/mol)) - (1 * 0 kJ/mol) - (3 * 0 kJ/mol)
ΔG° = -32.90 kJ/mol
Therefore, the ΔG° for the reaction N2(g) + 3H2(g) ⟶ 2NH3(g) at 298.15 K and a pressure of 12.52 mmHg for each gas is -32.90 kJ/mol.
Learn more about Gibbs free energy change here: brainly.com/question/13318988
#SPJ11
Which field of study examines homologous structures?
A) Genetics
B) Embryology
C) Comparative Anatomy
D) Physics
Answers
As the reaction in a galvanic cell proceeds towards products, which of the following are true?
A) ΔG starts at 0, stays same
B) ΔG starts < 0, becomes more negative
C) ΔG starts < 0, stays same
D) ΔG starts < 0, becomes more positive
E) ΔG starts > 0, stays same
Answers
In a galvanic cell, the reaction proceeds towards the production of products. ΔG starts < 0, becomes more negative
Option B is correct .
As the reaction proceeds, the Gibbs free energy (ΔG) reduces, and the following are true: ΔG starts < 0, becomes more negative.
When the reaction in a galvanic cell proceeds towards the production of products, the Gibbs free energy starts with a negative value, and it becomes even more negative.
The Gibbs free energy (ΔG) is a measure of the available energy in a system that can be used to do work. It measures the difference between the free energy of the final state and the initial state.The Gibbs free energy change of a system is dependent on the enthalpy and entropy change. If the enthalpy change is negative (exothermic), and the entropy change is positive (disorderly), the Gibbs free energy change is negative, and the reaction is spontaneous.
Learn more about Gibbs energy :
brainly.com/question/13765848
#SPJ11
During a thunderstorm mel saw lightning Flash in the distance a few seconds later mel Heard thunder Which of the following is reasonable explanation for Mel's observations?
Answers
During a thunderstorm men saw lightning Flash in the distance a few seconds later men heard thunder . So , during a thunderstorm , lightning is seen first and thunder is heard later on because light travels faster than sound .
Because sound travels at a speed of 330 m / s while light travels at a speed of 300,000,000 m / s , lightning appears earlier and thunder appears later . A sound cannot travel as quickly as light . As a result , you are the first to see the lightning's light .
Five times hotter than the sun's surface, the air in the lightning channel can get as hot as 50,000 degrees Fahrenheit. The air rapidly cools and constricts following the flash. The sound wave that we associate with thunder is produced by this quick expansion and contraction.
to learn more about lightning Flash please click here ,
https://brainly.com/question/14525716
#SPJ1
Summarize how the structure of organic compounds allows them to function as pigments in 2 – 3 sentences
Answers
The structure of organic compounds allows them to function as pigments through chromophore and acid or basic groups.
What is a Pigment?This is defined as a colored substance which is completely or nearly insoluble in water.
The structure of organic compounds allows them to function as pigments include chromophore and the acid or basic groups such as OH, SO3H, etc.
Read more about Pigment here vhttps://brainly.com/question/1056549
#SPJ1
when 0.367 mol of a weak acid, hx, is dissolved in 2.00 l of aqueous solution, the ph of the resultant solution is 2.60. calculate ka for hx. report your answer rounded to two significant figures using e- notation.
Answers
when 0.367 mol of a weak acid, hx, is dissolved in 2.00 l of aqueous solution, the ph of the resultant solution is 2.60. ka for hx is 3.405 × \(10^{-5}\)
Number of moles = 0.367
Volume of solution = 2l
concentration = 0.367/2 = 0.1835 mol/L
ph = 2.60
we know
ph = - log [H+]
2.51 × \(10^{-2.60}\)M = [H+]
The acid HX dissociate as
HX → H+ + X-
The acid dissociation constant Ka, for the dissociation reaction is
Ka = [H+][X-]/[HX] ; at equilibrium, [H+] = [X-]
Ka = 3.405 × \(10^{-5}\)
A solution in which water serves as the solvent is called an aqueous solution. The most common way to represent it in chemical equations is to add (aq) to the appropriate chemical formula. For instance, the formula for a solution of table salt, or sodium chloride (NaCl), in water is Na+(aq) + Cl The word aqueous, which derives from the word aqua, means that something is connected to, resembles, or is dissolved in water. Water is a common solvent in chemistry because it is an excellent solvent and abundant in nature. Since water is frequently used as the experiment's solvent, unless otherwise stated, the term "solution" refers to an aqueous solution.
Learn more about Aqueous solution here:
https://brainly.com/question/14097392
#SPJ4
I need help with Molar Ratio
______SO2(g) +_____O2(g) ------> _____SO3(g)
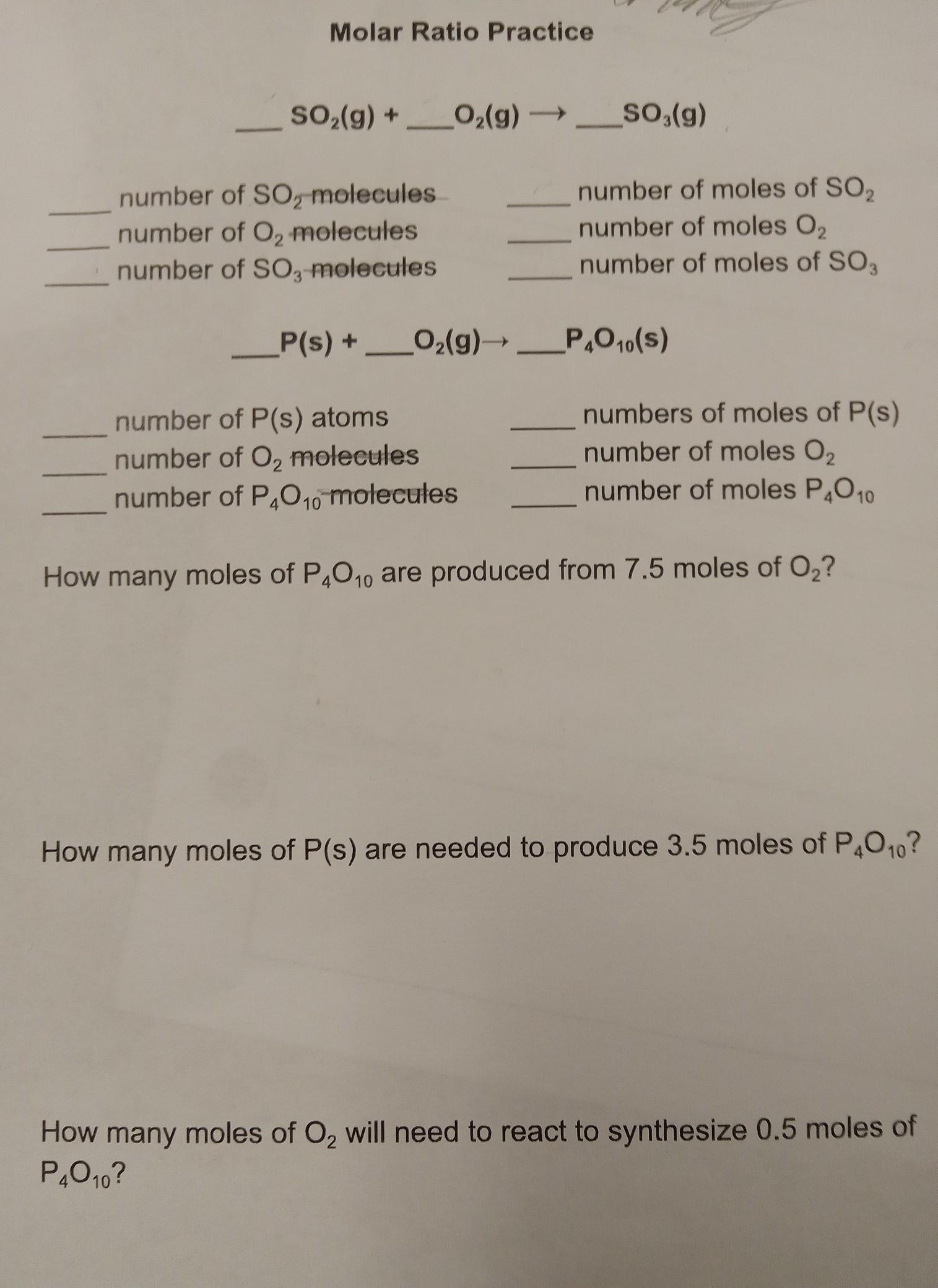
Answers
2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
1.204 * 10²⁴ number of SO₂ molecules = 2 number of moles of SO₂
6.02 * 10²³ number of O₂ molecules = 1 number of moles O₂
1.204 * 10²⁴ number of SO₃ molecules = 2 number of moles of SO,
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
2.408 * 10²⁴ number of P(s) atoms = 4 numbers of moles of P(s)
3.01 * 10²⁴ number of O₂ molecules = 5 number of moles O₂
6.02 * 10²³ number of moles P₄O₁₀ = number of P₄O₁₀ molecules
1.5 moles of P₄O₁₀ are produced from 7.5 moles of O₂.
14 moles of P(s) are needed to produce 3.5 moles of P₄O₁₀.
2.5 moles of O₂ will need to react to synthesize 0.5 moles of P₄O₁₀.
What is the mole ratio of the given reactions?The mole ratio of the given reactions is obtained from their equations of reaction.
1. 2 SO₂ (g) + O₂ (g) ---> 2 SO₃ (g)
The mole ratio is 2 : 1 : 2
1 mole of atoms or molecules contains 6.02 * 10²³ particles.
Hence, the number of particles is obtained by multiplying the number of moles by 6.02 * 10²³.
4 P(s) + 5 O₂ (g) ----> P₄O₁₀ (S)
7.5 moles of O₂ will produce 7.5/5 moles of P₄O₁₀ = 1.5 moles of P₄O₁₀
3.5 moles of P₄O₁₀ will be produced by 3.5 * 4 moles of + = 14 moles of P(s)
0.5 moles of P₄O₁₀ will be produced by 0.5 * 5 moles of O₂ = 2.5 moles of O₂
Learn more about mole ratio at: https://brainly.com/question/30632038
#SPJ1
How does a balanced chemical equation demonstrate the Law of Conservation of Mass?
(1 point)
A. It shows that all compounds remain bonded after the reaction
B. It shows that no atoms have been gained or lost during the reaction
C. It shows that the properties of the elements stay the same after the reaction
D. It shows that only physical changes follow the Law of Conservation of Mass
Thank you If you help!
Answers
the rays produced in a cathode tube are
Answers
Answer:
Electrons
Explanation:
Cathode rays carry electronic currents through the tube. Electrons were first discovered as the constituents of cathode rays. J.J. Thomson used the cathode ray tube to determine that atoms had small negatively charged particles inside of them, which he called “electrons.”
The rays produced in a cathode tube are electrons which are present in the shells surrounding the nucleus of an atom.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ6
Which is an example of a physical change?
gasoline combusting
cake baking
salt dissolving
iron rusting
Answers
Answer:
salt dissolving
Explanation:
Hope this helps!
Please can someone explain the differences in variables (e.g. control variable, Independent variable and Dependent variable). I know the definition, but I'm still not sure which is which when undertaking an experiment. Could someone possibly give me an example of an experiment and which is which?
Answers
Answer:
Independent Variable: The thing in an experiment which is changed.
Dependent Variable: The thing in an experiment which is affected by the change. (In other words, it's affected by the independent variable.)
Control Variable: The other factors of the experiment that remain unchanged.
For example, say you want to do an experiment about the effects of water on plant height. So, the independent variable, or the thing that is changed would be the amount of water, and the dependent variable would be the plant height, as the plant height is directly affected by the independent variable. Lastly, for this to be a fair experiment, there must be control variables. For example, all of the plants should be put in a room with equal temperature, they should get the same amount of sunlight, etc.
I hope this answers your question- feel free to ask me if you need any more help.
Answer with Explanation:
Lol.. even i was confused before .. so i learnt now ill help u :
Simple!
See... lets take simple expirament to explain:
My expirament is to verify if sunlight affects the growth of plants ...
So here :
My controlled variable : It is what is held constant.. So ur supposed to NOT it like everything else expect sunlight..(ex: water, soil, type of plant etc)
My Independent variable : Variable that is changed.... So this is wht u ARE changing to make th expirament ... (for ex: here sunlight u will chanhe... u will put on in a dark room and a nother in the light..)
My dependetn variabe:l Some thing that changes on is own..cause of the expiramen t... like the result ..(for ex: height , growth of plant etc)
I hope u understood !! :))
A warm air moist air mass moves over a cold air mass is most likely to lead to which type of server weather
Answers
Explain how inertia and gravity combine to keep earth on its orbit
Answers
Inertia and gravity combine to keep the Earth in its orbit around the Sun. Inertia is the tendency of an object to remain in motion or at rest unless acted upon by an external force.
Gravity is the force of attraction between two objects that have mass. The Sun's gravitational pull on the Earth creates a centripetal force that pulls the Earth towards the Sun. At the same time, the Earth's inertia is trying to keep it moving in a straight line. These two forces balance each other out, and as a result, the Earth orbits the Sun in an elliptical path. The amount of gravitational force between two objects depends on their masses and the distance between them. The larger the mass of the objects and the closer they are, the stronger the gravitational force. In the case of the Earth, the force of gravity exerted by the Sun is strong enough to overcome the Earth's inertia and keep it in its orbit.
To know more about gravity click here:
https://brainly.com/question/31321801
#SPJ11
GIVING BRAINLY PLEASE HELP ME!!

Answers
Answer:
1. Renewable Resource
2. Carbonate
3. Fossil Fuel
4. Nonrenewable Resource
5. Carbon Cycle
Answer:
1. Renewable Resource
2. Carbonate
3. Fossil Fuels
4. Non-renewable Resource
5. Carbon Cycle
6. 3 mL of 0.115 M KOH was needed to arrive at the equivalence point when 15.0 mL of HNO3 was titrated. What is the molarity of HNO3
Answers
To determine the molarity of HNO3, we can use the concept of stoichiometry and the volume and concentration information provided. The balanced chemical equation for the reaction between KOH and HNO3 is: KOH + HNO3 -> KNO3 + H2O
From the given information, we know that 3 mL of 0.115 M KOH is required to reach the equivalence point when titrating 15.0 mL of HNO3.
Using the concept of stoichiometry, we can set up a ratio between the volumes and concentrations of the two solutions:
(Molarity of KOH) x (Volume of KOH) = (Molarity of HNO3) x (Volume of HNO3)
(0.115 M) x (3 mL) = (Molarity of HNO3) x (15.0 mL)
Solving for the molarity of HNO3, we get:
Molarity of HNO3 = (0.115 M) x (3 mL) / (15.0 mL)
Molarity of HNO3 = 0.023 M
Therefore, the molarity of HNO3 is 0.023 M.
Learn more about molarity here;
brainly.com/question/31545539
#SPJ11
8. Which pair of substances react together?
A bromine and potassium chloride
B bromine and potassium iodide
C iodine and potassium bromide
D iodine and potassium chloride
Answers
Propane (c3h8) is a component of natural gas and is used in domestic cooking and heating. c3h8 5o2 --> 3co2 4h2o how many grams of carbon dioxide can be produced by burning 3.65 moles of propane? assume that oxygen is the excess reagent in this reaction.
Answers
Below is the balanced chemical equation. 1.07 103 kilograms of carbon dioxide will be created. Every balanced chemical equation adheres to the principle of mass conservation.
What does "balanced chemical equation" mean?A chemical equation is said to be balanced if the quantity of each type of atom in the reaction is the same on both the reactant and product sides. In a balanced chemical equation, the mass and the change are both equal.
Every balanced chemical equation follows the law of conservation mass , according to the information provided.
The total number of individual atoms on the reactant side and the total number of individual atoms on the product side must match,
according to this law.
The balancing chemical for the indicated reaction equation follows:
C₃H₈+5O₂→3CO₂=4H₂O
All the substances are present in gaseous state.
By Stoichiometry of the reaction:
1 mole of propane gas produces 3 moles of carbon dioxide gas.
So, 3.65 moles of propane gas will produce = of carbon dioxide gas.
Now, calculating the mass of carbon dioxide using equation:
3/1×3.65
=10.95
Molar mass of carbon dioxide = 44 g/mol
Moles of carbon dioxide = 24.33 mol
Putting values in above equation, we get:
10.95=Mass of carbon dioxide÷44g/mol
=4.50×10³g
Hence, the amount of produced in the given reaction and expressed in scientific notation is 4.50×10³
to know more about balanced chemical equation visit;
https://brainly.com/question/15352767
#SPJ4
i need help the is due tomorrow

Answers
Answer:
Bond
Explanation:
Bond is a link between atoms and molecules, and cohere means the uniting of something, so therefore it is bond.
which type of chemical bond occurs when atoms share electrons
Answers
When atoms share electrons, a covalent bond occurs.
A covalent bond is formed when atoms share electrons between them. In this type of chemical bond, atoms with unpaired electrons in their outermost energy levels (valence electrons) come together and share one or more pairs of electrons. Covalent bonding is commonly observed in nonmetallic elements or between nonmetallic and hydrogen atoms.
The shared electrons create a stable electron configuration for both atoms involved in the bond, resulting in a more stable molecule. The number of electrons shared determines the nature of the covalent bond. Single covalent bonds involve the sharing of one electron pair, double covalent bonds involve two pairs, and triple covalent bonds involve three pairs of electrons.
Covalent bonds are essential in many biological and chemical processes. They form the foundation of organic chemistry, where carbon atoms commonly form covalent bonds with other atoms. Covalent bonds contribute to the stability and structure of molecules, allowing for the formation of complex macromolecules like proteins, DNA, and carbohydrates.
learn more about Covalent bond here:
https://brainly.com/question/19382448
#SPJ11
select the steps that are associated with energy entering the system.
Answers
They include the folowing;
1. The break-up of firm particles = Endothermic heat absorbing process
2. The break-up of solute fragments = Endothermic heat arresting process
3. The joining of solute and solvent particles = Exothermic heat bearing process
The Enthalpy of Resolution maybe found in this manner including three elements: ΔHsoln = ΔH1 + ΔH2+ ΔH3
1. Break-up of the solute molecules from each one (extending the solute), this is an endothermic response. (ΔH1)
2. Break-up of the financially sound molecules from each one (extending the solid fragments), this is also an endothermic backlash (ΔH2)
In their divided states, the solute and fit fragments are free to attract each one liquid.
3. The exothermic response of the solute and fit resulting in the composition of the resolution. (ΔH3)
read more about energy entering the system
https://brainly.com/question/30279924
#SPJ4
Oxygen is not a large part of the air used by organisms in respiration therefore it is not an important element.
Please select the best answer from the choices provided
OT
OF
Answers
Answer
false
explanation
air is the most important element in respiration
Which of the following statements about the equilibrium constant, Keq is true? O When Ke>1, the equilibrium favors the reactants. O When Kea1, the equilibrium favors the products. O The size of Keg tells about the position of equilibrium. O For a reaction to be useful, the equilibrium must favor the reactants.
Answers
The correct statement about the equilibrium constant, Keq, is that when Keq>1, the equilibrium favors the products.
The Keq value gives an idea about the relative concentration of reactants and products at equilibrium. If Keq is greater than 1, it means that the concentration of products is more than the concentration of reactants, and the equilibrium favors the products.
Conversely, if Keq is less than 1, it means that the concentration of reactants is more than the concentration of products, and the equilibrium favors the reactants. The magnitude of Keq also gives an idea about the extent of the reaction, i.e., the larger the Keq value, the more the reaction goes towards completion. However, it does not tell us about the speed of the reaction.
More on equilibrium constant: https://brainly.com/question/28559466
#SPJ11
10 While a student is holding a piece of metal in her hand, her hand gets colder. What happens to the temperature of the metal? * m (6 Points) A. The piece of metal will get warmer because some thermal energy is transferred from the metal to the student's hand. B. The piece of metal will get warmer because some thermal energy is transferred from the student's hand to the ON metal. C. The piece of metal will stay at the same temperature because an equal amount of thermal energy is exchanged between the student's hand and the metal. D. The piece of metal will stay at the same temperature because thermal energy is not transferred between the student's hand and the metal.
Answers
Answer:
B
Explanation:
thermal energy is the energy between one object to another
What type of reaction does this model represent?
1. single replacement
2. double replacement
3. Decomposition
4. double displacement

Answers
Answer:
1. single replacement
Explanation:
Single replacement can be represented by AB+C ⇄B+AC, which matches the picture
Double replacement and double displacement are the same kind of reaction and can be represented by AB+CD ⇄BD+AC
Decomposition reaction can be represented by AB ⇄A+B