Answers
Answer:
2Al+ 6HCl -->2AlCl3 + 3H2
2NaCl --> 2Na + 1Cl2
1Na2S + 2HCl --> 2NaCl + 1H2S
CH4 + 2O2 --> CO2 + 2H2O
2Na + 1Cl2 --> 2NaCl
2H2 + O2 --> 2H2O
Related Questions
If a piece of cadmium with a mass of 37.60 g and a temperature of 100.0 oC is dropped into 25.00 cc of water at 23.0 oC, what will be the final temperature of the system
Answers
Answer:
\(T_{eq}=28.9\°C\)
Explanation:
Hello!
In this case, since it is observed that hot cadmium is placed in cold water, we can infer that the heat released due to the cooling of cadmium is gained by the water and therefore we can write:
\(Q_{Cd}+Q_{W}=0\)
Thus, we insert mass, specific heat and temperatures to obtain:
\(m_{Cd}C_{Cd}(T_{eq}-T_{Cd})+m_{W}C_{W}(T_{eq}-T_{W})=0\)
In such a way, since the specific heat of cadmium and water are respectively 0.232 and 4.184 J/(g °C), we can solve for the equilibrium temperature (the final one) as shown below:
\(T_{eq}=\frac{m_{Cd}C_{Cd}T_{Cd}+m_{W}C_{W}T_{W}}{m_{Cd}C_{Cd}+m_{W}C_{W}}\)
Now, we plug in to obtain:
\(T_{eq}=\frac{37.60g*0.232\frac{J}{g\°C}*100.00\°C+25.00g*4.184\frac{J}{g\°C}*23.0\°C}{37.60g*0.232\frac{J}{g\°C}+25.00g*4.184\frac{J}{g\°C}}\\\\T_{eq}=28.9\°C\)
NOTE: since the density of water is 1g/cc, we infer that 25.00 cc equals 25.00 g.
Best regards!
For a chemical reaction in a closed system, mass Connor be _____ or _______. We can say that throughout the reaction mass is ______.
Answers
Answer:
conserved or created/destroyed. We can say that throughout the reaction mass is conserved.
For a chemical reaction in a closed system, mass Connot be or destroyed . We can say that throughout the reaction mass is conserved .
During a chemical reaction, atoms are rearranged and bonded together in new combinations, forming different substances. However, the total number of atoms remains the same. This means that the total mass of the reactants must be equal to the total mass of the products.
The law of conservation of mass is based on the principle that atoms are neither created nor destroyed during a chemical reaction. Instead, they are rearranged and redistributed into different chemical species.
It is important to note that while mass is conserved, the substances involved in the reaction may undergo changes in physical state (solid, liquid, gas) or experience changes in energy, such as the release or absorption of heat. These changes do not affect the total mass of the system.
In summary, for a chemical reaction in a closed system, mass cannot be created or destroyed. The law of conservation of mass states that the total mass of the reactants is equal to the total mass of the products, and throughout the reaction, mass is conserved.
This principle is a fundamental concept in chemistry and plays a crucial role in understanding and balancing chemical equations.
For more such questions on chemical reaction visit:
https://brainly.com/question/25769000
#SPJ8
what is the name of ch3cooc2h5
Answers
Answer:
ethyl ethanoate
Explanation:
it helps you
23 What predictions can you make about the melting point and
boiling point of krypton, which is the next gas in this group?
Answers
Explanation:
Answer:krypton toh superman ko maarta haiExplanation:no
in an investigation that uses the scientific method which step immediately follows asking a question
Answers
Answer:
Infer and form a hypothesis
No FILES PLZZ
What can all non-renewable resources theoretically be?
A
converted to nonmetallic minerals
B
converted to renewable ones
exhausted or depleted
D
recycled or reused
Answers
Answer:
B.
Converted to renewable ones
exhausted or depleted
What is the molarity of Ca(NO3)2 in a solution resulting from mixing 150.0 mL of 0.200 M HNO3 with 150.0 mL of 0.0100 M Ca(OH)2
Answers
The molarity of the Ca(NO3)2 solution would be 0.005 M
First, let us look at the balanced equation of the reaction:
\(2HNO_3+Ca(OH)_2 ---> Ca(NO_3)_2+2H_2O\)
The mole ratio of HNO3 to Ca(OH)2 is 2:1.
Mole of HNO3 = molarity x volume
= 0.2 x 150/1000
= 0.03 mole
Mole of Ca(OH)2 = 0.01 x 150/1000
= 0.0015
Thus, there is no limiting or excess reactant.
Also from the equation, mole ratio of Ca(OH)2 to Ca(NO3)2 is 1:1. Hence, the mole of Ca(NO3)2 would also be 0.0015 mole.
The total volume of the resulting solution would be: 150 +150 = 300 mL
Thus, the molarity of the resulting Ca(NO3)2 would be:
Molarity = mole/volume
= 0.0015/0.3
= 0.005 M
More on molarity can be found here: https://brainly.com/question/12127540
3.
What are valence electrons? How do you quickly determine the number of valence electrons an
atom has?
Answers
Answer:
Electrons that arent electrons
Explanation:
I teach chem
Answer:
Valence electrons are the electrons found in the highest and main energy level of the atom, being these responsible for the interaction between atoms of different species or between atoms of the same species. You can quickly determine these by looking at a periodic table like this one... Which is where the roman numbers come in and show the valence electrons. (THIS TABLE IS IN SPANISH, I hope you still recognize the symbols.) :))

neutral particles called?
Answers
Answer:
Neutron
Explanation:
hope it helps and your day will be full of happiness
Which of the following is the best explanation for melting? Circle or highlight the correct choice. A. Particles heat up, losing kinetic energy, and spread further apart so they are less attracted. B. Particles heat up, gaining kinetic energy, and spread further apart so they are less attracted. C. Particles cool down, losing kinetic energy, and get closer so they are more attracted. D. Particles cool down, gaining kinetic energy, and get closer so they are more attracted.
Answers
all the living and nonliving things in an specific area
Answers
Answer: An Ecosystem
Explanation:
Select all of the following options that must be done in case of a fire alarm or other evacuation (when feasible).
a. gather your belongings
b. turn off all hot plates & other heating apparatus
c. turn off all water
d. stay in the lab to finish your experiment
Answers
option a,b,c are correct.gather your belongings,turn off all hot plates & other heating apparatus, turn off all water these are options that must be done in case of a fire alarm or other evacuation .
To guarantee your safety, take prompt action when the fire alarm goes off. The fire alarm system was created and constructed to give you a head start so you may safely leave the building in an emergency.Never dismiss a warning or believe it to be fake or the outcome of a test.The nearest and safest exit and/or stairs must be used by everyone to leave the building.Never leave during a fire alarm activation in an elevator.Once outside, make your way away from the structure. Gather on the sidewalk of the building next door or across the street.The firefighters and fire engines will be working in front of the structure. Don't stand in their way as they enter the building.
learn more about heating Refer:/brainly.com/question/11737047
#SPJ4
what is the pH of a solution that contains 0.0425 moles of HCl in 6.50 litres of water?
Answers
Answer:
To find the pH of a solution that contains a strong acid like HCl, you can use the equation:
pH = -log[H+]
Where [H+] is the concentration of hydrogen ions in moles per liter. In this case, you know the concentration of HCl and the volume of water, so you can calculate the concentration of hydrogen ions by using the equation:
[H+] = (0.0425 moles HCl) / (6.50 liters water) = 0.0653846 moles/liter
Finally, you can calculate the pH:
pH = -log[0.0653846 moles/liter] = 1.18
So the pH of the solution is 1.18.
Explanation:
Do you want to know shot trick also?
Draw the structure of phosphatidylserine and discuss its components
Answers
Phosphatidylserine is a type of phospholipid that is mainly found in cell membranes. Its structure is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule.
The fatty acid chains are hydrophobic, meaning they repel water, while the phosphate group and serine molecule are hydrophilic, meaning they attract water.
The glycerol molecule acts as a bridge that connects the two fatty acid chains to the phosphate group and serine molecule.
The structure of phosphatidylserine is important for its function in the cell membrane.
Because of the hydrophobic and hydrophilic components of its structure, phosphatidylserine is able to form a lipid bilayer, which is a barrier that separates the inside of the cell from the outside environment.
The hydrophilic heads of the phosphatidylserine molecules face outward and interact with water, while the hydrophobic tails face inward and repel water.
Phosphatidylserine also plays a role in cell signaling and apoptosis, which is programmed cell death.
It acts as a signaling molecule by binding to proteins that are involved in cellular pathways.
In addition, phosphatidylserine is translocated to the outer leaflet of the cell membrane during apoptosis, which signals to immune cells that the cell is ready to be removed.
In conclusion, the structure of phosphatidylserine is made up of two fatty acid chains, a phosphate group, a serine molecule, and a glycerol molecule. Its hydrophobic and hydrophilic components allow it to form a lipid bilayer in cell membranes, and it also plays a role in cell signaling and apoptosis.
For more such questions on Phosphatidylserine
https://brainly.com/question/16179573
#SPJ8
In an isolated system, two copper bars at different temperatures transfer energy until both are at the same temperature. How would the transfer of
energy be different if the bars were in an open system?
OA Energy transfer would occur only between the copper bars.
OB. Energy transfer would occur between the copper bars and the surroundings.
OC. No energy transfer would occur between the copper bars or the surroundings.
OD. Energy transfer would occur only with the surroundings.
Answers
The manner in which the transfer of energy would be different if the bars were in an open system is as follows: Energy transfer would occur between the copper bars and the surroundings (option B).
What is law of conservation of energy?The law of conservation of energy principle stating that energy may not be created or destroyed.
An isolated system exchanges neither energy nor matter with the surroundings. According to this question, two copper bars at different temperatures transfer energy until both are at the same temperature.
However, in an open system, some of the energy would be transferred to the surroundings.
Learn more about energy transfer at: https://brainly.com/question/13087586
#SPJ1
how is gas different from a liquid and a solid
a. a gas is mad of tiny particles
b. a gas has volume
c. a gas expands to fill the container
d. a gas has density
Answers
Answer:
a. a gas is mad of tiny particles
Explanation:
I believe
What is the formula for Decaoxygen pentasulfide
Answers
Answer:
Compound Formula. 1, Carbon dioxide, CO2. 2, Carbon monoxide, CO. 3, Diphosphorus pentoxide, P2O5. 4, Dinitrogen monoxide, N2O.
Explanation:
dont even know by thoAnswer:
Explanation:
(HELP PLEASE) which pair would have an electrostatic force of attraction between them?
Na+ and Ar
Cs+ and Rb+
Cl− and O 2−
K+ and P 3−
Answers
Answer: K+ and P 3−
Explanation:
Electrostatic force of attraction is the attraction between different molecules that comes from them being electrically charged.
For molecules to have an electrostatic force of attraction, they need to have opposing charges because like charges repel each other but opposite charges attract.
In the question above, the only option that has opposing charges is option D so this is the correct option.
On the original series, MacGyver was a television character that could build things from everyday objects, frequently using chemistry and his knowledge of the many trends in the periodic table. What are two of the trends that are found in the periodic table? What is responsible for each of these trends occurring within the periodic table? Use this information to explain what part of the periodic table would contain the best elements to use from the trend that you selected
Answers
Answer:
The periodic trends are patterns that we can find in the periodic table that show specific properties. Those properties nclude metallic character, non metallic character, atomic radius, ionization energy or electronegativity.
If we have to select two we can work with: electronegativity and atomic radius.
Electronegativity:
The electronegativity is a chemical property that describes the ability of an atom to attract electrons. It's useful when determining if a bond between two atoms is covalent or ionic.
Across the periodic table the electronegativity increases from left to right and from bottom to top. We can see the values in the next table and identify that trend.
Elements on the right side of the table almost have their valence shell complete, they can easily gain electrons to become stable. Their electronegativiy is high. On the other hand, the elements on the left side of the period table only have 1 (group 1) or 2 (group 2) valence electrons. It's easier for them to lose electrons, so their electronegativity is low.
Atomic radius:
The atomic radius is one-half the distance between the nuclei of two atoms.
Atomic size decreases from left to right across a a period of elements. In the same period all the electrons are added to the same shell. As the atomic number increases (from left to right) we are adding one proton to the nucleus and one electron to the shell. As the number of protons in the nucleus increases, the nucleus becomes more positivily charged and the nucleus attracts the electrons more strongly. The electrons in the shells are closer to the nucleus, so the size of the atom or the atomic radius decreases.
The atomic size or the atomic radius increases from top to bottom. Down a group the valence electrons occupy higher levels of energy and the valence electrons are further away from the nucleus. As a result the atomic radius is larger as we go down a group.

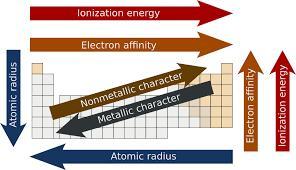
PLZ HELP 40 POINTS PLEASEEEEEE

Answers
Answer:
should be A
Explanation:
A student was asked to determine the concentration of ammonia, a volatile substance used in the clinical setting as a respiretory stimulant to prevent fainting. First the student pipetted 25.00 mL of the cloudy ammonia solution into a 250.0 mL conical flask. 50.00 mL of 0.100 mol L' HCl(aq) was immediately added to the conical flask which reacted with the ammonia in solution. The excess (unreacted) HCI was then titrated with 0.050 mol L- Na2CO3(aq). 21.50 mL of Na2CO3(aq) was required. Calculate the concentration of the ammonia in the cloudy ammonia solution.
Answers
This question is describing two chemical equations whereby the concentration of ammonia has to be determined. The first reaction is between 25.00 mL of ammonia and 50.00 mL of 0.100-M HCl whose excess was neutralized with 21.50 mL of 0.050-M Na₂CO₃ and thus, the concentration ammonia in the cloudy solution was determined as 0.114 M.
First of all we need to go over the titration of the excess HCl with Na₂CO₃ by writing the chemical equation it takes place when they react:
\(2HCl+Na_2CO_3\rightarrow 2NaCl+CO_2+H_2O\)
Whereas the mole ratio of HCl to Na₂CO₃ is 2:1 and the volume of the HCl leftover is determined as follows:
\(V_{HCl}^{leftover}=\frac{2*0.050M*21.50mL}{0.100M} =21.5mL\)
Next, we infer that the consumed volume of HCl by the ammonia solution was:
\(V_{HCl}^{consumed}=50.00mL-21.50mL=28.5 mL\)
Then, we write the chemical equation that takes place between ammonia and HCl:
\(HCl+NH_3\rightarrow NH_4Cl\)
Whereas the mole ratio is now 1:1, which means that the concentration of ammonia was:
\(M_{NH_3}=\frac{28.5mL*0.100M}{25.00mL}\\\\ M_{NH_3}=0.114M\)
Learn more:
(Titration) https://brainly.com/question/15687419(Titration) https://brainly.com/question/25328286If your equation includes 7(CrO4)2, how many Cr's are there?
If your equation includes 7(CrO4)2, how many O's are there?
Answers
If an equation includes 7(CrO₄)₂, the numbers of Cr's and O's atoms that are there are 14 and 56 respectively.
How to calculate number of atoms?The number of atoms present in a chemical compound can be calculated by multiplying the subscript of the particular element by any coefficient.
According to this question, 7 moles of chromate with the chemical formula; (CrO₄)₂ is given. The number of oxygen and chromium atoms in this compound can be calculated as follows:
Chromium = 7 (coefficient) × 2 = 14 atomsOxygen = 7 (coefficient) × 8 = 56 atomsLearn more about no of atoms at: https://brainly.com/question/14190064
#SPJ1
does anyone know thisss???

Answers
consider the balanced chemical equation below. when the chemical reaction was carried out calculated theoretical was yield for sodium bromide 162 grams but the measured yield was 150 grams what is the percent yield?
Answers
Answer:
Explanation:
% yield = (actual yield / theoretical yield) X 100
For this question,
% yield = (150g/ 162 g) X 100 = 92.6%
Answer:
92.6%
Explanation:
You are taking a scuba diving training course. The instructor is discussing
your scuba tanks, and tells you they have a volume of about 11 L and hold
enough gas for a one-hour dive. The instructor also tells you that you take in
about 2 L of gas with each breath. Which is the best explanation for why 11 L
of gas lets you breathe for one hour?
A Because of the high pressure of the water, the amount of gas
needed for each breath decreases.
B It is very cold underwater. This decreases the pressure inside the
tank and allows exhaled gases to be stored and breathed in
again.
© The gas is under pressure in the tank. As pressure increases,
volume decreases. A small tank can therefore hold a large
amount of gas.
Underwater the gas becomes cold enough for it to become a
solid. The solid vaporizes a little at a time to allow you to breathe
for longer
Answers
Answer:
C
Explanation:
The deeper you go into water, the greater pressure there is on everything. This compresses the air inside and causes the volume to shrink.
Sometimes air is measured in bars. Let's assume the Earth's surface pressure is 1. Let's also say that your lungs have 5.5L and the tank has 11L. That's only 2 breathes, but under 200 bars of pressure deep in the ocean? That's 200 x 11, which equals 2200L. That's about 400 breathes.
In addition, if making liters out of thin air doesn't make any sense, there's an alternative where you divide your liters per breath by the bar pressure. So instead of 200 x 11, you can take your 5.5 from your lungs and divide it by 200. That small number, in this case, 0.0275, can serve as how much air you breath deep in the ocean.
What best describes the dropping height of a ball that bounced back up to a height of 45 centimeters?
Less than 45 centimeters, as the ball transforms some of its thermal energy into potential energy
Greater than 45 centimeters, as the ball transforms some of its thermal energy into potential energy
Less than 45 centimeters, as the ball transforms some of its potential energy into thermal energy and sound energy
Greater than 45 centimeters, as the ball transforms some of its potential energy into thermal energy and sound energy
Answers
Answer:
less than 45 cm, as the ball transforms some of its potential eneregy to termal energy and sound energy
a student has a 1 L solution of 2 M HCL and wants to increase the HCL concentration to 3 M
Answers
The student needs to add approximately 83.3 mL of 12 M HCl solution to the existing 1 L of 2 M HCl solution to increase the concentration to 3 M. It is important to handle concentrated acids with caution and follow proper safety procedures.
To increase the concentration of a 1 L solution of 2 M HCl to 3 M, the student needs to calculate the volume of concentrated HCl needed and add it to the existing solution. Here's how the calculation can be done:
Given:
Initial concentration of HCl solution = 2 M
Final concentration desired = 3 M
Initial volume of HCl solution = 1 L
Step 1: Calculate the moles of HCl in the initial solution.
Moles of HCl = Initial concentration × Initial volume = 2 M × 1 L = 2 moles
Step 2: Calculate the moles of HCl needed for the desired concentration.
Moles of HCl needed = Final concentration × Final volume = 3 M × 1 L = 3 moles
Step 3: Calculate the moles of HCl to be added.
Moles of HCl to be added = Moles needed - Moles present = 3 moles - 2 moles = 1 mole
Step 4: Convert the moles of HCl to the required volume of concentrated HCl.
To calculate the volume, we need to know the concentration of the concentrated HCl solution. Assuming it is 12 M, we can use the following formula:
Volume of concentrated HCl = Moles of HCl to be added / Concentration of concentrated HCl
Volume of concentrated HCl = 1 mole / 12 M = 0.0833 L or 83.3 mL
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
I have a balloon with an initial temperature of 22 degrees Celcius and a volume of 5.50 liters. What will be the volume of the balloon when it is fully cooled by my refrigerator? The temperature inside my refrigerator is about 4 degrees Celcius.
Answers
The volume of the balloon is 12 it reaches this then it is fully cooled by my refrigerator.
What is volume ?
Space is occupied by every three-dimensional object. Its volume serves as a gauge for this area. The area contained by an object's limits in three-dimensional space is referred to as its volume. Another name for it is an object's capacity.
What is temperature ?
The movement of these particles likewise increases with rising temperature. A thermometer or a calorimeter are used to measure temperature.
Therefore, The volume of the balloon is 12 it reaches this then it is fully cooled by my refrigerator.
Learn more about volume from the given link.
https://brainly.com/question/463363
#SPJ1
Physical methods of monitoring the rate of a chemical reaction
Answers
There are several physical methods that can be used to monitor the rate of a chemical reaction are; Spectrophotometry, Conductometry, and Turbidity measurement
Spectrophotometry involves measuring the changes in the intensity of light absorbed or transmitted by a solution during a chemical reaction. Spectrophotometers are used to measure the amount of light absorbed or transmitted by a sample at different wavelengths.
Conductometry involves measuring the changes in electrical conductivity of a solution during a chemical reaction. Conductivity meters are used to measure the electrical conductivity of a solution, which can change as the concentration of ions in the solution changes during a chemical reaction.
Turbidity measurement involves measuring the changes in the clarity or turbidity of a solution during a chemical reaction. Turbidimeters or nephelometers can be used to measure the amount of light scattered by a sample, which can change as particles form or dissolve during a reaction.
To know more about Spectrophotometry here
https://brainly.com/question/31440604
#SPJ1
--The given question is incomplete, the complete question is
"What are the physical methods of monitoring the rate of a chemical reaction?"--
Which element will have a higher electronegativity?
A. Fluorine
B. Hydrogen
C. Bromine
D. Phosphorus
Answers
Answer:
fluorine is the most electronegative element,
Explanation:
while francium is one of the least electronegative. (Helium, neon, and argon are not listed in the Pauling electronegativity scale, although in the Allred-Rochow scale, helium has the highest electronegativity.)
Answer:
A. Fluorine
Explanation:
It said that according to periodic law that the group seven elements are highly electronegativity and in reduce down the group
Which mean that it is Fluorine
