if energy can neither be created nor destroyed, into what kinds of energy have these resources been transformed?
Answers
Answer:
Heat, Sound
Explanation:
I am pretty sure you are addressing mechanical energy and machine efficiency. So you are probably aware a machine can't achieve 100% efficiency because of the first and second laws of thermodynamics. This energy is lost to friction and air resistance. They convert some gravitational potential energy to heat and sound energy rather than all to kinetic energy, and vice versa.
Related Questions
189.77 Kelvin is equal to
°C.
Answers
-83.38 degrees celsius
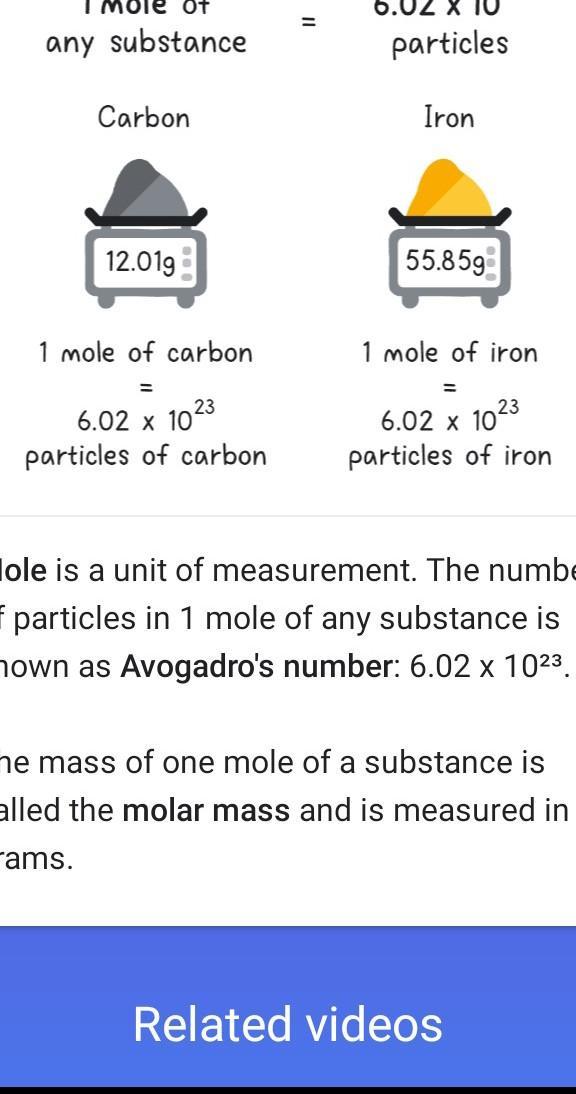
If you have a mole of boron and a mole of carbon would they have the same number of
atoms in each mole? Please help ❤️
Answers
Answer:. that contains the same number of entities as there are atoms in 12 g of carbon-12. ... the same as the mass of one mole of the compound in grams. • Skill 3-1 Calculate the ... Molar mass has units of grams per mole (g/mol). Concept 3. ... of moles to mass: •We can do the reverse with 1/M, and convert any mass in grams to.
Explanation:
The number of atoms in a mole of boron and carbon is required.
A mole of carbon and a mole of boron have the same number of atoms.
The unit of amount of substance is called mole.
\(1\ \text{mole}=6.022\times 10^{23}\ \text{atoms, molecules, ions, or electrons}\)
So, 1 mole of any element, whether it be carbon or boron has the same number of atoms which is \(6.022\times 10^{23}\).
Learn more:
https://brainly.com/question/20484279
https://brainly.com/question/1731823
What is the advantage of using multiple lines in a line graph
Answers
The lines in a line graph aid in the fairly early detection of trends, allowing the viewer to make predictions about the data that has not yet been recorded.These are appropriate for illustrating the data points since, given one variable, the other is simple to ascertain.
What is a line graph?A line graph, often known as a line chart, is a visual representation of data that is continuously changing over time.
There are two axes. in a line graph, the occurrences and categories being compared throughout time are shown on the x-axis (abscissa), and the scale, which is a group of integers that reflects the data and is divided into equal intervals, is shown on the y-axis (ordinate).
Depending on the data, the graph's lines may either decline or ascend. It is practical to plot time graphs along the x/y axis since it shows the rise and fall of data points in great detail.
The reader may readily see changes in one group over time by choosing the appropriate scales for each axis (time on the x-axis and change is measured on the y-axis).
To refer more about line graph, refer the link below:
https://brainly.com/question/23680294
#SPJ1
\
Beryllium's atomic mass is 9, and its atomic number is 4. How many protons are found in a beryllium atom?
Answers
Answer:
4 protons
Explanation:
The number of protons in an atom is equal to the atomic number. In this case, we have beryllium whose atomic number is 4, so it has 4 protons.
A carrot was cut into many pieces, and moved into a freezer what change is it
Answers
Answer:
Physical change
Explanation:
Cutting and freezing are both examples of physical changes that do not alter the chemical composition or properties of the object/substance.
Answer:
physical change
Explanation:
carrot lost its original shape
8) Hospitals buy 400 L cylinders of oxygen gas compressed at 150 atm pressure. They need to
administer oxygen to patients at 3.0 atm. What volume (V2) of oxygen can a cylinder supply at this lower
pressure?
Answers
Answer:
the answer will be 20,000 L
The diagram shows changes of state between solid,liquid,and gas.the atoms of a substance lose energy during a change of state.before the change,the atoms are close together but are able to slide past one another
Answers
Answer:
Q
Explanation:
I took the test.
A sample of certain gas have Volume of 1.25 L ATM _125 degree Celsius and5.0 ATM the gas is compressed 50.0 ATM a volume of 325 mL. what is final temperature?
Answers
The final temperature of the gas is approximately 40.96 Kelvin.
To determine the final temperature of the gas, we can use the ideal gas law, which states:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature in Kelvin.
First, let's convert the given temperatures to Kelvin. We have:
Initial temperature: -125 degrees Celsius = 148 K (approximate)
Final temperature: Unknown
The initial conditions of the gas are as follows:
Initial pressure (P1) = 1.25 atm
Initial volume (V1) = 1250 mL = 1.25 L (since 1 L = 1000 mL)
Initial temperature (T1) = 148 K
The final conditions of the gas are as follows:
Final pressure (P2) = 50.0 atm
Final volume (V2) = 325 mL = 0.325 L
Final temperature (T2) = Unknown
Using the ideal gas law, we can set up the following equation:
(P1 * V1) / T1 = (P2 * V2) / T2
Substituting the known values:
(1.25 atm * 1.25 L) / 148 K = (50.0 atm * 0.325 L) / T2
Simplifying the equation:
T2 = (50.0 atm * 0.325 L * 148 K) / (1.25 atm * 1.25 L)
T2 = 40.96 K
For more such questions on temperature visit;'
https://brainly.com/question/4735135
#SPJ8
someone anyone give me tips to finish online homework because i have 30 assignments to do
Answers
Answer:
Ok! I have four tips!
Explanation:
Create A Time Table.
Gather Information For Your Online Assignments Beforehand.
Make Notes As You Read The Course Materials.
Start Doing Your Online Assignment In Advance.
How can a student collect and measure the volume of a gas produced ?
Answers
The volume of oxygen produced can be measured using the gas syringe method. The gas collects in the syringe, pushing out against the plunger. The volume of gas that has been produced can be read from the markings on the syringe.
The specific heat capacity of liquid water is 4.18 J/g-K. How many joules of heat are needed to raise the temperature of 5.00 g of water from 15.0 °C to 36.5 °C?
Answers
The specific heat capacity of a substance is the amount of energy required to raise the temperature of 1g of the substance by 1K.
\(\begin{gathered} q=mc\Delta T \\ q:energy\text{ }(J)=x \\ m:mass\text{ }(g)=5.00g \\ c:specific\text{ }heat\text{ }capacity\text{ }(Jg^{-1}K^{-1}) \\ \Delta T:change\text{ }in\text{ }temperature\text{ }(K) \\ \Delta T:(final\text{ }temperature-initial\text{ }temperature) \end{gathered}\)Calculating the change in temperature:
\(\Delta T:(273.15K+36.5\degree C)-(273.15K+15\degree C)=21.5K\)By substituting what we are given into the equation to solve for the unknow x we have;
\(\begin{gathered} q=5.00g\times4.18Jg^{-1}K^{-1}\times21.5K \\ q=+449.35J \end{gathered}\)Answer: Energy needed is 449.35J
-. SEP Identify Patterns How did you classify reaction 4? Based on periodic
patterns (the available electrons to form bonds), provide an explanation for the
sodium chloride product formed.

Answers
CaCO3 + 2NaCl → CaCl2 + Na2CO3, this is a double displacement reaction.
What is double displacement reaction?Type of chemical reaction in which reactant ions exchange places to form new products is called as double displacement reaction.
The chemical equation for the reaction between calcium carbonate (CaCO3) and sodium chloride (NaCl) is: CaCO3 + 2NaCl → CaCl2 + Na2CO3
This is a double displacement reaction, where the calcium ion (Ca2+) and the sodium ion (Na+) switch partners to form calcium chloride (CaCl2) and sodium carbonate (Na2CO3).
Sodium (Na) and chlorine (Cl) are both in halogen group in periodic table, which implies they both have 7 electrons in the outermost energy level. This makes them highly reactive and they also tend to form ionic bonds with other elements to complete the outermost shell with 8 electrons. In the reaction between calcium carbonate and sodium chloride, sodium ion (Na+) and chloride ion (Cl-) form an ionic bond to produce sodium chloride (NaCl) as the product.
To know more about double displacement reaction, refer
https://brainly.com/question/23918356
#SPJ1
Enter the net ionic equation, including phases, for the reaction of agno3(aq) and kcl(aq). Refer to the solubility rules as needed.
Answers
The net equation is:
AgNO3(aq) + KCl(aq) ==> AgCl(s) + KNO3(aq) .
Chemical equations are symbols and chemical formulas that depict a chemical reaction symbolically. With a plus sign separating the entities in both the reactants and the products and an arrow pointing in the direction of the products to indicate the direction of the reaction, the reactant entities are given on the left and the product entities on the right. Chemical formulas can be mixed, structural (represented by pictures), or both. The absolute values of the stoichiometric numbers are shown as coefficients next to the symbols and formulas of the entities.
To know more about chemical equation, click here,
brainly.com/question/26694427
#SPJ4
If you have 500 ml of a 0.10 m solution of the acid, what mass of the corresponding sodium salt of the conjugate base do you need to make the buffer with a ph of 2.08 (assuming no change in volume)
Answers
The mass of the corresponding sodium salt of the conjugate base needed to make a buffer with a pH of 2.08.
To determine the mass of the corresponding sodium salt of the conjugate base needed to make a buffer with a pH of 2.08, you can follow these steps:
1. Identify the given information:
- Initial volume of acid solution: 500 mL
- Initial concentration of acid solution: 0.10 M
- Desired pH: 2.08
2. Use the Henderson-Hasselbalch equation:
pH = pKa + log ([conjugate base]/[acid])
3. Assuming the acid is a weak monoprotic acid (HA) and its conjugate base is A-, determine the pKa:
pKa = pH - log ([A-]/[HA])
4. Calculate the ratio of [A-] to [HA]:
[A-]/[HA] = 10^(pH-pKa)
5. Calculate the moles of HA in the 500 mL of 0.10 M solution:
moles of HA = (volume x concentration) = 500 mL x 0.10 mol/L = 0.050 mol
6. Calculate the moles of A- needed:
moles of A- = moles of HA x ([A-]/[HA]) ratio
7. Determine the molar mass of the sodium salt of the conjugate base (A-) using the molecular formula.
8. Calculate the mass of the sodium salt of the conjugate base:
mass = moles of A- x molar mass of A-
By following these steps, you will be able to determine the mass of the corresponding sodium salt of the conjugate base needed to make a buffer with a pH of 2.08.
To know more about conjugate base refer here: https://brainly.com/question/30086613#
#SPJ11
a 28.6 mass % aqueous solution of iron(iii) chloride has a density of 1.280 g/ml. calculate the molality of the solution. give your answer to 2 decimal places.
Answers
The molality of a 28.6 mass % aqueous solution of iron(III) chloride with a density of 1.280 g/mL is 2.67 mol/kg.
To calculate the molality, first find the mass of the solution and the mass of the solute (iron(III) chloride) and solvent (water). Since the density is 1.280 g/mL, the mass of 100 mL of the solution is 128 g (100 mL x 1.280 g/mL). In this solution, 28.6% is iron(III) chloride, so the mass of the solute is 36.61 g (0.286 x 128 g), and the mass of the solvent (water) is 91.39 g (128 g - 36.61 g).
Next, determine the moles of iron(III) chloride in the solution. The molar mass of iron(III) chloride (FeCl3) is 162.2 g/mol. Thus, there are 0.225 moles of iron(III) chloride in the solution (36.61 g / 162.2 g/mol).
Finally, calculate the molality by dividing the moles of solute by the mass of the solvent (in kilograms). Molality = 0.225 mol / 0.09139 kg = 2.463 mol/kg, which can be rounded to 2.67 mol/kg to two decimal places.
Summary: The molality of a 28.6 mass % aqueous solution of iron(III) chloride with a density of 1.280 g/mL is 2.67 mol/kg.
Learn more about molality click here:
https://brainly.com/question/1370684
#SPJ11
Every atom of the __ carbon has six protons
Answers
When sulfuric acid is combined with sugar, gas is released and a tall, black column forms. This is an example of a-
Answers
When sulfuric acid is combined with sugar, gas is released and a tall black column forms. This is an example of a Dehydration Reaction.
A Dehydration reaction is defined as a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions is the reverse of a hydration reaction. Concentrated sulfuric acid can perform a dehydration reaction with table sugar. After mixing, the color changes from white to brownish and eventually to black. The expansion of the mixture is the result of vaporization of water and CO2 inside the container. The gases inflate the mixture to form a snake-like shape and give off a burned sugar smell. The granularity of the sugar can greatly affect the reaction. powdered sugar reacts very quickly but sugar cubes take longer to react.
To learn more about Dehydration Reaction please visit:
https://brainly.com/question/29262706
#SPJ4
solid m2co3 dissolves until a saturated solution is formed where m is a certain metal ion. this solution has a m concentration of 0.062. determine the value of ksp. enter your answer in scientific notation with two significant figures. for example 0.001357 would be entered as 1.4e-3.
Answers
The value of Ksp for the dissolution of \(M_{2} CO_{3}\) is 1.2\(e^{-4}\)
The following formula can be used to represent the solubility product constant, Ksp, for the dissolution of solid \(M_{2} CO_{3}\) in water:
2\(M^{+}\) (aq) \(M_{2} CO_{3}\) (s) + \(CO_{3}\) (aq)
The dissolving reaction has the following equilibrium expression:
Ksp= \([M^{+} ]^{2}\) \([CO_{3} ]^{2-}\)
It is said that there are 0.062 M of \(M^{+}\) in the saturated solution. Therefore,
[\(M^{+}\) ] = 0.062 M
The initial concentration of \(M_{2} CO_{3}\) can be represented in terms of [\(M^{+}\)] as follows since \(M_{2} CO_{3}\) dissociates to give 2 \(M^{+}\) ions for each formula unit:
[\(M_{2} CO_{3}\)] = (0.062 M) / 2 = 0.031 M
Due to the fact that one \(CO_{3} ^{2-}\) ion forms for every two \(M^{+}\) ions, the concentration of \(CO_{3} ^{2-}\) ions is also 0.031 M at equilibrium. In light of this, the Ksp can be determined as follows:
Ksp = \([M^{+} ]^{2}\) \([CO_{3} ]^{2-}\) = \((0.062 M)^{2}\) (0.031 M) = 1.2 x \(10^{-4}\) = 1.2\(e^{-4}\)
To know more about ksp,
https://brainly.com/question/4736767
#SPJ4
What is the temperature and pressure at STP?
Answers
Answer:
Since 1982, STP is defined as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of exactly 105 Pa (100 kPa, 1 bar).
Explanation:
I hope I helped
The bacteria are known to be prokaryotes. Prokaryote means
A
organisms whose cells have no nucleus
B
organisms who are made of many cells
C
organisms who make their own food
D
organisms whose cells have a nucleus

Answers
Answer:
prokaryote, also spelled procaryote, any organism that lacks a distinct nucleus and other organelles due to the absence of internal membranes. ... Bacteria are among the best-known prokaryotic organisms. The lack of internal membranes in prokaryotes distinguishes them from eukaryotes.
Explanation:
A 10.0-g sample of krypton has a temperature of 25 °C at 563 mmHg. What is the volume, in milliliters, of the krypton gas?
Express your answer to three significant figures and include the appropriate units.
Answers
The volume, in milliliters, of the krypton gas is 523ml.
The Ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. The ideal gas equation can be written as
PV = nRT
where,
P = Pressure
V = Volume
T = Temperature
n = number of moles
Given,
Mass = 10g
Pressure = 563 mm Hg
Temperature = 298 K
moles of Kr =mass / atomic mass
= 10 / 84
= 0.119 moles
PV = nRT
563 × V = 0.119 × 8.314 × 298
V = 0.523L = 523ml
Learn more about Ideal Gas law, here:
https://brainly.com/question/28257995
#SPJ1
10 To draw a perfect circle, you'll need: A A protractor B. A sextant С A compass D A spirograph 10 of 10
Answers
Answer:
B
Explanation:
Two quick google searches later I grace you with the google has bestowed upon me
Animals in an experiment are to be kept under a strict diet. Each animal should receive 25 grams of protein and 5grams of fat. The laboratory technician is able to purchase two food mixes: Mx A has 10% protein and 6% fat; mix B has 50% protein and 5% fat. How many grams of each mix should be used to obtain the right diet for one animal? One animar's diet should consist of grams of MaA.
Answers
250 grams of Mix A (MxA) should be used to obtain the right diet for one animal.
To determine the number of grams of Mix A (MxA) needed to obtain the right diet for one animal, let's assume that x represents the number of grams of MxA used.
The protein content in MxA is 10%, which means 0.10x grams of protein will be obtained from MxA.
The fat content in MxA is 6%, which means 0.06x grams of fat will be obtained from MxA.
Since the desired diet for one animal should consist of 25 grams of protein and 5 grams of fat, we can set up the following equation based on the protein content:
0.10x = 25
Solving for x:
x = 25 / 0.10
x = 250 grams.
To know ore about protein
brainly.com/question/31017225
#SPJ11
A hypothesis does not need to be correct for an experiment to be successful because
a hypothesis is an educated guess about what will happen in an experiment.
True
Or
False
Answers
Given the density of 1.2 g/mL and a volume 7.8 mL. What is the mass? Round to the nearest 0.1.
Answers
Answer:
here is my answerrrrrrr

at stp what is the volume of 5.35 moles of methane ch4
Answers
Answer:
22.4 L
Explanation:
Need help with this.

Answers
Answer:
18.2 g.
Explanation:
You need to first figure out how many moles of nitrogen gas and hydrogen (gas) you have. To do this, use the molar masses of nitrogen gas and hydrogen (gas) on the periodic table. You get the following:
0.535 g. N2 and 1.984 g. H2
Then find out which reactant is the limiting one. In this case, it's N2. The amount of ammonia, then, that would be produced is 2 times the amount of moles of N2. This gives you 1.07 mol, approximately. Then multiply this by the molar mass of ammonia to find your answer of 18.2 g.
How many grams are in 1.5 moles of P? Select one:
a. 23 g
b. 46.5 g
c. 47 g
d. 22.5 g
Answers
Explanation:
I think the answer is B ♂️ but I'm not sure
CsH16 +12028CO2 +8H₂O
What is the ratio of octene (C8H16) to
oxygen in the reaction?
Answers
The ratio of octene to oxygen is 1:12.
To determine the ratio of octene (C8H16) to oxygen (O2) in the given reaction, we need to examine the balanced chemical equation. However, the equation you provided does not seem to be balanced. The coefficients for each compound must be determined to achieve a balanced equation before we can calculate the desired ratio.
Assuming you meant the combustion reaction of octene, a balanced equation would be:
C8H16 + 12O2 → 8CO2 + 8H2O
From the balanced equation, we can see that for every 1 mole of octene (C8H16), we require 12 moles of oxygen (O2) to completely react.
This means that for every 1 mole of octene, we need 12 moles of oxygen to fully combust the octene and produce the corresponding amounts of carbon dioxide (CO2) and water (H2O) as shown in the balanced equation.
For such more questions on combustion
https://brainly.com/question/13251946
#SPJ8
what three factors determine if something is pollutant?
Answers
Answer:
They include: Chemical nature of the pollutant - how active and toxic it is to humans, plants and animals; Concentration - the amount of the chemical per unit volume of air, water, soil, and; Persistence or longevity - how long the pollutant remains in the environment in its harmful form.
Explanation:
Air pollutants like ozone, particulate matter, carbon monoxide, nitrogen oxide, sulfur dioxide, lead cause atmospheric pollution which regards to ozone depletion, GH effect, climate change and human health effects. If anyone say the main factors of air pollution beyond of industry, means of transportation, solid waste generation and urbanization.
Growing urbanization and industrialization grows more pollution. Due to a large number of population in the world, more pollution is generated. Specifically if we talk about air pollution, due to steady increase in number of industries, the gradient level is deteriorating day by day. There is no good if we just talk about sustainable development if we are not following it.
Answer:
Explanation:
Chemical nature of the pollutant ConcentrationPersistence