ionic compounds are formed from ionic bonds, whereby an electron is transferred from the metal cation to the nonmetal anion. ions form solid lattices of ions. covalent compounds form solids through the attraction of two covalent molecules. since the attraction between two covalent molecules is weak compared to the ionic bonds holding an ionic compound together, ionic compounds tend to have higher melting points. which of the following compounds has the highest boiling point? view available hint(s)for part c ionic compounds are formed from ionic bonds, whereby an electron is transferred from the metal cation to the nonmetal anion. ions form solid lattices of ions. covalent compounds form solids through the attraction of two covalent molecules. since the attraction between two covalent molecules is weak compared to the ionic bonds holding an ionic compound together, ionic compounds tend to have higher melting points. which of the following compounds has the highest boiling point? f2 naf hf clf
Answers
Ionic Compounds have high boiling point compare to covalent bon due to presence of electrostatic force.
Ionic bonds, also known as electrovalent bonds, are created in chemical compounds when ions with different charges attract one another electrostatically. When the valence (outermost) electrons of one atom are permanently transferred to another atom, a bond of this sort is created. If an atom receives electrons, it generates a negatively charged ion (cation), and if it loses electrons, it forms a positively charged ion (cation) (anion).
The best examples of this sort of chemical are those created when alkali and alkaline-earth metals are combined with nonmetals. Ionic bonding results in the formation of electrovalent or ionic molecules. The electrostatic forces of attraction between opposing charges and repulsion between similar charges are what keep the ions in these specific types of ionic crystalline solids aligned.
know more about Ionic Compounds visit : https://brainly.com/question/9167977
#SPJ4
Related Questions
Octane, a compound of hydrogen and carbon, has a molar mass of 114.26 g per mole. If one mole of the compound contains 18.17 g of hydrogen what is the molecular formula
Answers
The compound octane is a hydrocarbon with the molar mass of 114.26 g/mol. It contains 8 carbon atoms and 18 hydrogens. Hence, the molecular formula is C₈H₁₈.
What is octane ?Hydrocarbons are organic compounds containing carbon -hydrogen bonds. Alkanes are saturated hydrocarbons with the general formula of CₙH₂ₙ₊₂.
Given the molar mass of octane = 114.26 g/mol
mass of hydrogen = 18.17 g.
atomic mass of H = 1.009 g/mol
then, number of moles of H = 18.17 /1.009 = 18 moles.
Thus, there are 18 hydrogens.
Now, the mass of carbons = 114.26 -18.17 = 96.6 g
atomic mass of carbon = 12 g/mol
no.of moles of carbon = 96.6/12 = 8 moles
Thus, there are 8 carbons.
Therefore, the molecular formula of octane is C₈H₁₈.
Find more on octane:
https://brainly.com/question/17457251
#SPJ1
How many dimes could you trade for 360 pesos? $1 = 1500 pesos.
O 1.5 dimes
O 5.4 dimes
O 540 dimes
O 2.4 dimes
Answers
Answer:
The correct option is (d).
Explanation:
It is given that,
1$ = 1500 pesos
We need to convert 360 pesos into dimes
We can convert 360 pesos to dollars as follows:
\(360\ \text{pesos}=\$\dfrac{1}{1500}\times 360\\\\=$0.24\)
360 pesos is equal to $0.24
Also, 1 dollar = 10 dimes
We can covert 0.24 dollar to dimes as follows :
0.24 dollar = 10 × 0.24 dimes
0.24 dollar = 2.4 dimes
or
360 pesos = 2.4 dimes
a reaction has a δhrxn = 23.25 kj and δs was 161.26 j/mol∙k. this reaction is spontaneous
Answers
The spontaneity of a reaction is determined by the Gibbs free energy change (∆G), which is a measure of the system's ability to do work.
The equation that links ∆G, ∆H, and ∆S is: ∆G = ∆H - T∆S, where T is the temperature in Kelvin and ∆H and ∆S are the enthalpy and entropy changes, respectively. The signs of ∆H and ∆S determine whether the reaction is exothermic or endothermic and whether it is entropy-driven or enthalpy-driven, respectively.
If ∆G is negative, the reaction is spontaneous, meaning it occurs without the input of energy.The given reaction has a ∆H of 23.25 kJ and a ∆S of 161.26 J/mol∙K.
First, we need to convert the units of ∆S from J/mol∙K to kJ/mol∙K by dividing by 1000.∆S = 161.26 J/mol∙K ÷ 1000 = 0.16126 kJ/mol∙K Substitute the values into the equation to determine the spontaneity of the reaction:
∆G = ∆H - T∆S∆G = (23.25 kJ) - (298 K) x (0.16126 kJ/mol∙K)∆G = 23.25 kJ - 48.02 kJ∆G = -24.77 kJ Since ∆G is negative, the reaction is spontaneous.
To know more about Gibbs free energy refer here: https://brainly.com/question/13795204#
#SPJ11
How much energy is required to raise the temperature of 3 kg of lead from 15°C to 20°C? Use the table below and this equation: Q = MCAT.
The question is written right above the table given.
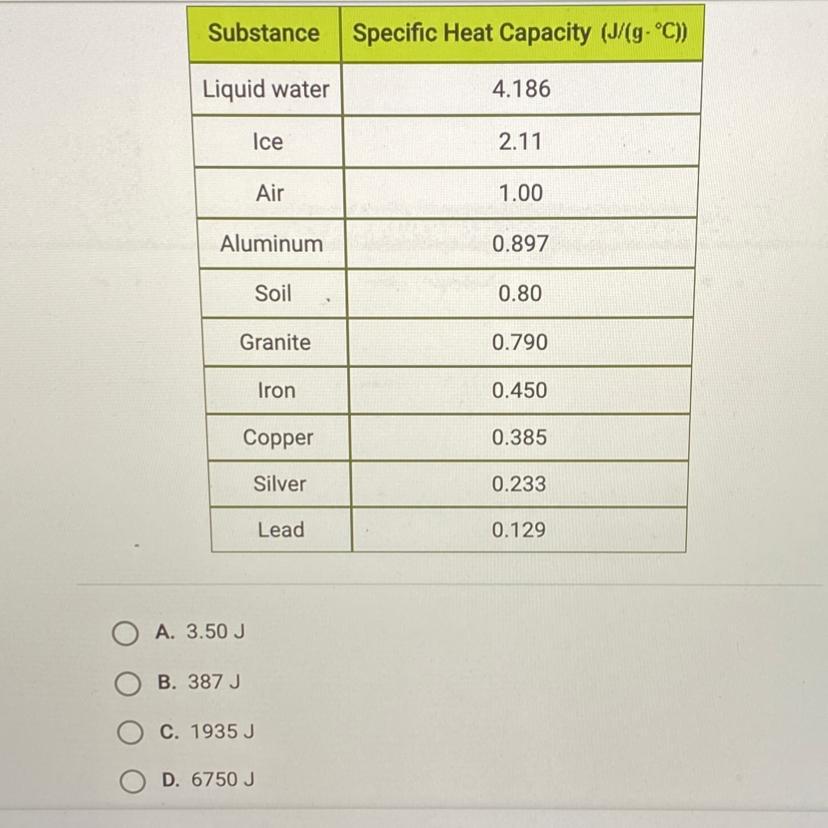
Answers
Answer:
1935J
Explanation:

Answer:
\(\boxed {\boxed {\sf C. \ 1935 \ J}}\)
Explanation:
The equation for this problem is:
\(q=mc\Delta T\)
where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
The mass is 3 kilograms, but the specific heat capacity includes grams in the units. Convert kilograms to grams. There are 1000 grams in 1 kilogram.
\(\frac {1000 \ g}{1 \ kg}\)\(3 \ kg *\frac {1000 \ g}{1 \ kg}\)\(3 *1000 \ g = 3000 \ g\)The specific heat capacity for lead is found on the table. It is 0.129 J/g°C.
Let's find the change in temperature. It is raised from 15 °C to 20 °C.
\(\Delta T= final \ temperature - initial \ temperature \\\Delta T= 20 \textdegree C - 15 \textdegree C\\\Delta T= 5 \textdegree C\)Now we know every value.
m= 3000 g c= 0.129 J/g°CΔT= 5 °CSubstitute the values into the formula.
\(q= (3000 \ g)( 0.129 \ J/g \textdegree C)(5 \textdegree C)\)
Multiply the first 2 numbers together. The units of grams cancel.
\(q= (387 \ J/ \textdegree C )(5 \textdegree C)\)
Multiply again. This time the units of degrees Celsius cancel.
\(q= 1935 \ J\)
1935 Joules of energy are required and choice C is correct.
A 45.0 cm3 sample of gas is heated from 280. K to 315 K. What is the new volume of the gas sample?
Answers
Answer:
50.63 cm³
Explanation:
Using Charles law equation as follows:
V1/T1 = V2/T2
Where;
V1 = initial volume (cm³)
V2 = final volume (cm³)
T1 = initial temperature (K)
T2 = final temperature (K)
According to the question, V1 = 45.0 cm³, V2 = ?, T1 = 280 K, T2 = 315 K
Using; V1/T1 = V2/T2
45/280 = V2/315
Cross multiply
45 × 315 = 280 × V2
14175 = 280V2
V2 = 14175 ÷ 280
V2 = 50.625
V2 = 50.63 cm³
What is the mass of a sample of material that has a volume of 55.10
cm^3 and a density of 6.720 g/cm^3?
Answers
Answer:
The answer is 370.27 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
volume = 55.10 cm³
Density = 6.720 g/cm³
The mass is
mass = 55.10 × 6.720 = 370.272
We have the final answer as
370.27 gHope this helps you
Why is only increasing the ph of the ocean not enough to restore the ocean’s equilibrium?.
Answers
The increasing pH of an ocean is not enough to restore the equilibrium as seawater becomes more acidic, and the pH of the water drops. This process binds carbonate ions, reducing their abundance.
What is the ocean's equilibrium?Equilibrium is a state where the presence of base and acid is in equal quantity.
Increased pH, leads to more acidity and reduces the number of ions.
Ions are necessary for corals, oysters, mussels, and many other shelled organisms that require building their shells and skeletons.
Thus, increasing pH of an ocean is not enough to restore the equilibrium as seawater becomes more acidic, and the pH of the water drops. This process binds carbonate ions, reducing their abundance.
Learn more about equilibrium
https://brainly.com/question/13463225
#SPJ1
I SWEAR THIS IS WAY TOO EASY, WHY AM I THAT STUPID, HELP ME AND ILL MARK U BRAINLIEST

Answers
Answer:
i think the first one is gravity and second one is rotation
Explanation:
draw the structure of two geometric isomers with the empirical formula c5h8o that give a positive iodoform test.
Answers
The positive iodoform test indicates the presence of a methyl ketone or a compound that can undergo oxidation to form a methyl ketone. In the case of C5H8O, two geometric isomers that can give a positive iodoform test are trans-2-pentene-1-ol and cis-3-penten-2-ol. Here are their structures:
Trans-2-pentene-1-ol:
H
|
H - C = C - C - C - OH
| |
H H
Cis-3-penten-2-ol:
H
|
H - C = C - C - OH
| |
H H
Both of these isomers have the empirical formula C5H8O and can undergo oxidation to form a methyl ketone, which will react with iodine and hydroxide ions to produce a yellow precipitate of iodoform.
It's important to note that the structures provided are examples of geometric isomers that fit the given empirical formula and can give a positive iodoform test. The actual arrangement of atoms in space may vary depending on the specific isomer.
learn more about geometric isomers
https://brainly.com/question/31744339?referrer=searchResults
#SPJ11
One mole is a quantity unit that is always equal to __________________________.
Answers
Answer:
6.022 × 10²³ units of that substance
Explanation:
will % recovery be impacted if you do not wait for the recovered crystals to dry before weighing them? what about the melting point? explain your reasoning clearly.
Answers
Yes, the % recovery will be impacted if you do not wait for the recovered crystals to dry before weighing them. The melting point, however, will not be affected.
When determining the % recovery of a substance, it is important to accurately measure the mass of the recovered product. If the crystals are not allowed to dry completely before weighing, they may still contain traces of moisture, which can add to their mass and lead to an overestimation of the recovery percentage.
Moisture can be absorbed from the surrounding environment, especially if the crystals are hygroscopic (have a tendency to attract and retain moisture).
To obtain an accurate % recovery, it is crucial to ensure that the crystals are completely dry before weighing them. This can be achieved by allowing them to air dry or using techniques such as vacuum drying or desiccation with drying agents.
On the other hand, the melting point of a substance is determined by the temperature at which it changes from a solid to a liquid state. The presence of moisture within the crystals, if not fully dried, may affect the melting point determination due to the vaporization of water at lower temperatures. However, once the crystals are completely dry, their melting point should remain unaffected.
In summary, while the % recovery can be impacted by not waiting for the crystals to dry before weighing, the melting point is not affected as long as the crystals are fully dry during the measurement.
To know more about melting point refer here:
https://brainly.com/question/25777663#
#SPJ11
shown below is a dipeptide. a. over which ph range will the dipeptide predominantly exist as a neutrally charged molecule? b. what is the isoelectric point of this peptide? show your calculations to support your answer.
Answers
a. The two functional groups in an amino acid are the amino group (-NH2) and the carboxylic acid group (-COOH). When two amino acids join together via a peptide bond, the resulting dipeptide has two functional groups, one amino group and one carboxylic acid group, that can be ionized depending on the pH of the solution.
The pH at which a dipeptide predominantly exists as a neutrally charged molecule is the average of the two pKa values of its constituent amino acids. In this case, one amino acid has a pKa of 2.34 for the carboxylic acid group and a pKa of 9.60 for the amino group, while the other amino acid has a pKa of 2.20 for the carboxylic acid group and a pKa of 9.13 for the amino group.
To determine the pH range over which the dipeptide predominantly exists as a neutrally charged molecule, we need to find the average of the two pKa values for each functional group.
For the carboxylic acid group:
(pKa1 + pKa2) / 2 = (2.34 + 2.20) / 2 = 2.27
For the amino group:
(pKa1 + pKa2) / 2 = (9.60 + 9.13) / 2 = 9.37
Therefore, the pH range over which the dipeptide predominantly exists as a neutrally charged molecule is around pH 2.27 to pH 9.37.
b. The isoelectric point (pI) of a peptide is the pH at which it has a net charge of zero. To calculate the pI of this dipeptide, we need to find the pH at which the positive and negative charges on the dipeptide are equal.
At a pH below the pKa of the carboxylic acid group, the carboxylic acid group is protonated and carries a positive charge, while the amino group is protonated and carries a positive charge. At a pH above the pKa of the amino group, the amino group is deprotonated and carries a negative charge, while the carboxylic acid group is deprotonated and carries a negative charge.
Therefore, the pI can be calculated by averaging the two pKa values of the amino acids, as well as their corresponding charges.
pI = (pKa1 + pKa2) / 2 = (2.34 + 2.20 + 9.60 + 9.13) / 4 = 5.32
The isoelectric point of this dipeptide is 5.32.
To know more about dipeptide reactions, visit the link given below:
https://brainly.com/question/6194023
#SPJ4
Circle the letter of each sentence that is true about what can occur in a chemical change.
a. Compounds may be broken down into elements.
b. Elements may combine to form compounds.
c. Compounds may change from one state to another.
d. Compounds may change into other compounds.
Answers
Answer:
b
Explanation:
The statement that is true about what can occur in a chemical change is b. Elements may combine to form compounds.
What is chemical change?A chemical change can be described as the change which do take place when the substance's composition is been altered which could bring about the formation of another substance.
It should be noted that in the chimical change there is uaually a formation of a new product after one or more substance must have reacted together which implies that in this kind of change there is usuall a new substance that will be formed in the process.
Learn more about chemical change at:
https://brainly.com/question/1222323
#SPJ1
Copper turns a green-brown when it is exposed to oxygen in air. What chemical property of oxygen causes this effect? A. its reactivity B. its volume C. its mass D. its flammability
Answers
Answer:
A. its reactivity
Explanation:
It's reactivity because copper was exposed to air and if it is reactivity it must be exposed to air
Answer:
A. reactivity
Explanation:
Question 1
A) How can a stationary metal sphere have kinetic energy, the energy of motion? (1 point) (A
The metal's molecules are moving around.
The metal is made of atoms, which store potential energy.
The metal sphere can be rolled.
x?
The metal is made of atoms, which are vibrating in place.
Lectuo sol orit éttornslew orit bruos vis eff
absolute heat of the substance
Question 3
?
(
Question 2
A) What do you measure when you find a substance's temperature? (1 point)
average kinetic energy of the particles in the substance Xadhoesb teed ons
Okinetic energy of the substance
Opotential energy of the substance
vlleutnavs tem evewle feum soduo Bat
2 noitesuo
easier thermal energy to the app
01 Briw vijos sedmem rope grillet .mset e diw ashow are
65 y transis/ thermal energy to the corn
grumoheq vodnem roso rillw,89 no arow or
gy, so they transfer thermal energy to the roon.
losjong erti tuo viso bus rose2a1 of encls zahow orl2
mothy as the spoctis holecules, so there
emae ort to lis tuo onlyreo redimem das dily meat a no ashow ar
à noitesuo
A) What happens when thermal energy is applied to an ice cube? (1 point)
OIts water molecules gain potential energy. to noleuionpo ori to noilghozeb lead on ci tortW (A
Its water molecules lose kinetic energy, so the ice cube melts. Xener esau noleutdinog gitt
1
Its water molecules lose potential energy.aniteoval prit vidauj of princess apsu noleutonos or
Its water molecules gain kinetic energy and move around more.
Xart asteta noteutonoo ont
nettholed notion deevnt er to opta fall sitt el holautongo siff
(10
Answers
The stationary metal sphere can have kinetic energy by: The metal's molecules are moving around.
When you find a substance's temperature, you are measuring the "average kinetic energy of the particles in the substance." Temperature is a measure of the average kinetic energy of the particles (atoms or molecules) that make up a substance.
What is kinetic energy?Kinetic energy is the energy an object possesses due to its motion. It is defined as the energy that a body possesses by virtue of being in motion. The amount of kinetic energy that an object has depends on its mass and its velocity. Mathematically, the kinetic energy of an object can be expressed as KE = (1/2)mv^2, where KE is the kinetic energy, m is the mass of the object, and v is its velocity.
Read more on kinetic energy here:https://brainly.com/question/8101588
#SPJ1
A scuba diver 40 ft below the ocean surface inhales 95.0 mL of compressed air from a scuba tank at a pressure of 2.90 atm and a temperature of 9 ∘C. What is the pressure of the air, in atm , in the lungs when the gas expands to 195.0 mL at a body temperature of 37 ∘C , and the amount of gas remains constant?
Answers
1.553 atm is the pressure of the air, in atm , in the lungs when the gas expands to 195.0 mL at a body temperature of 37 ∘C , and the amount of gas remains constant.
V1=95 ml
P1=2.90 atm
T1=282 K
V2=195 ml
T2=310 K
by applying ideal gas equation
P1V1÷T1=P2V2÷T2
P2=P1×V1×T2÷V2×T1
P2= 85405 ÷54990
P2=1.553 atm
The definition of pressure is force/area. For instance, the weight of the snow divided by the area of the roof would represent the pressure from snow on a roof. Gases are often where pressure in chemistry originates from. Vacuum is the term for the lack of pressure. Since "nature abhors a vacuum," humans have believed that vacuums are both impossible and unnatural for hundreds of years. This isn't really the case.
The amount of pressure units is insane! The torr or mmHg is a common unit. Simply put, this refers to the height of a mercury column. The atmospheric pressure is about 760 torr, or mmHg. You may also see mmH2O, which employs the same notion, but in this instance the atmospheric pressure is around 10.3 mH2O since water is less dense than mercury.
To know more about pressure visit : https://brainly.com/question/18124975
#SPJ1
A popular radio station has a frequency of 93.3 MHz. What is the length of this radio
wave in meters?
7.8 m
8.5 m
3.2 m
O 4.5 m
6.3 m
Answers
The length of this radio wave is approximately 3.2 meters. So, the correct answer is option (C) 3.2 m.
The speed of light in a vacuum is approximately 3.00 x 10^8 m/s. The relationship between the frequency of a wave (f) and its wavelength (λ) is given by:
c = fλ
where c is the speed of light. Solving for the wavelength, we get:
λ = c/f
Substituting the given values, we get:
λ = (3.00 x 10^8 m/s)/(93.3 x 10^6 Hz)
λ = 3.22 meters
For more question on radio wave click on
https://brainly.com/question/29690463
#SPJ11
Which of the following items describe a mole? a. Avogadro's number of items b. 6.022 multiply 10^23 items c. mass multiply acceleration d. The amount of a substance containing the same number of formula units as there are atoms in 12 g of carbon.
Answers
The correct descriptions of a mole are:
a. Avogadro's number of items
b. 6.022 × \(10^2^3\) items
d. The amount of a substance containing the same number of formula units as there are atoms in 12 g of carbon.
a. Avogadro's number of items:
Avogadro's number is a fundamental constant in chemistry and is defined as the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. It is approximately equal to 6.022 × \(10^2^3\) items. Therefore, option a correctly describes a mole as Avogadro's number of items.
b. 6.022 × \(10^2^3\) items:
This is the numerical value of Avogadro's number. As mentioned earlier, it represents the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. So, option b is another correct description of a mole.
c. Mass multiplied by acceleration:
This description does not accurately describe a mole. The product of mass and acceleration is a measure of force (Newton's second law of motion) and is unrelated to the concept of a mole in chemistry.
d. The amount of a substance containing the same number of formula units as there are atoms in 12 g of carbon:
This is a correct description of a mole. It refers to the concept of the molar mass, where one mole of a substance contains the same number of particles (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. This concept allows for the conversion between mass (in grams) and the number of moles.
So, the correct options are a, b, and d.
To know more about Avogadro's number, refer
here:https://brainly.com/question/28812626
#SPJ4
hydrogen bonds are select one: a. weak chemical bonds that hold together atoms within a molecule. b. strong chemical bonds that hold together atoms within a molecule. c. weak chemical bonds that hold separate molecules together. d. strong chemical bonds that hold separate molecules together.
Answers
Hydrogen bonds are (c) weak chemical bonds that hold separate molecules together.
Not to be confused with a covalent link to a hydrogen atom, hydrogen bonding is a unique form of dipole-dipole attraction that occurs between molecules. It comes about as a result of the attraction between two extremely electronegative atoms, such as N, O, or F, and a hydrogen atom that is covalently bound to one of them.
Although hydrogen bonds individually are weak and easily broken, they are present in very high quantities in water and organic polymers, where they combine to provide a powerful force. The DNA double helix is held together by hydrogen bonds as well.
You can also learn about hydrogen bond from the following question:
https://brainly.com/question/15099999
#SPJ4
The diagram shows the water cycle. At point 4, condensation is occurring. How is water changing?
A) Water vapor (gas) is becoming liquid.
B) Water is being stored in the atmosphere.
C) Liquid water in the atmosphere is becoming a gas.
D) Liquid water from the earth's surface is becoming water vapor (gas)
Answers
Answer:
water vapor (gas) is becoming liquid
What does the answer of element 3Na2S2O3?
Answers
Answer:
Explanation:
3× 22+2×32+ 6×16=
66+64+96=236
Jen is walking her dog at a constant rate.
They keep a constant rate as they turn a
corner. Why has their velocity changed?

Answers
Answer:
4is the answer
Explanation:
1.Calculate the mass of anhydrous sodium tetraoxosulphate (VI) present in 500cm3 of 0.5M.
1b.The number of Na2SO4 particles present in the solution. (Na=23, S=32,O=16)
Answers
There are approximately 1.5055 ×\(10^{23\) \(Na_2SO_4\)particles present in the solution.
To calculate the mass of anhydrous sodium tetraoxosulphate (VI) (\(Na_2SO_4\)) present in 500 cm3 of 0.5 M solution, we can use the equation:
Mass (g) = Volume (L) × Concentration (M) × Molar mass (g/mol)
First, let's convert the volume from cm3 to L:
500 cm3 = 500/1000 L = 0.5 L
Next, we can substitute the values into the equation:
Mass = 0.5 L × 0.5 mol/L × (2 × 23 g/mol + 32 g/mol + 4 × 16 g/mol)
= 0.5 × 0.5 × (46 + 32 + 64)
= 0.5 × 0.5 × 142
= 35.5 g
Therefore, the mass of anhydrous sodium tetraoxosulphate (VI) present in 500 cm3 of 0.5 M solution is 35.5 g.
For 1b, to calculate the number of \(Na_2SO_4\)particles present in the solution, we can use Avogadro's number (6.022 ×\(10^{23\) particles/mol) and the number of moles of Na2SO4.
The number of moles of Na2SO4 can be calculated using the formula:
Moles = Concentration (M) × Volume (L)
Moles = 0.5 mol/L × 0.5 L
= 0.25 mol
Now we can calculate the number of \(Na_2SO_4\)particles:
Number of particles = Moles × Avogadro's number
= 0.25 mol × 6.022 ×\(10^{23\) particles/mol
= 1.5055 × \(10^{23\) particles
For more such questions on particles visit:
https://brainly.com/question/31213916
#SPJ8
How many atoms would you find in 3.60 g of Boron?
Answers
Answer:
1.8 * 10^24 atoms
Explanation:
n = M(B) / m = 10.8 / 3.60 = 3 mol
N = n * Na = 3 * 6*10^23 = 1.8 * 10^24 atoms
The density of a liquid is determined by successively weighing 25, 50, 75, 100, and 125 mL of the liquid in a 250-ml beaker. If volume of liquid is plotted along the horizontal axis, and total mass of beaker plus liquid is plotted on the vertical axis: Select one:
a. The x, or horizontal, intercept is the negative value of the weight of the beaker.
b. The slope of the line is independent of the identity of the liquid.
c. The y, or vertical, intercept is the weight of the empty beaker,
d. The line will pass through the origin.
e. The slope of the line is 1.0.
Answers
C. The y, or vertical, intercept is the weight of the empty beaker.
When determining the density of a liquid, it is important to use a specific container to hold the liquid. In this case, a 250-ml beaker was used. The method used to determine the density involves weighing 25, 50, 75, 100, and 125 mL of the liquid in the beaker. By plotting the volume of liquid along the horizontal axis and the total mass of beaker plus liquid on the vertical axis, it is possible to analyze the results. Option a is incorrect, as the x-intercept does not represent the weight of the beaker. It is important to note that the beaker's weight should be measured and subtracted from the total mass obtained from the experiment. Option b is also incorrect because the slope of the line is dependent on the identity of the liquid being used.
Option c is correct, as the y-intercept represents the weight of the empty beaker. This value is crucial in determining the total mass of the liquid. Finally, option d is incorrect because the line will not pass through the origin, and option e is also incorrect because the slope of the line will vary depending on the identity of the liquid. In summary, the correct answer is c.
learn more about density Refer: https://brainly.com/question/29775886
#SPJ11
A double-replacement reaction takes place when aqueous Na2CO3 reacts with aqueous Sn(NO3)2. You would expect one of the products of this reaction to be?
Answers
One of the products of this reaction is solid tin(II) carbonate (SnCO₃).
When aqueous sodium carbonate (Na₂CO₃) reacts with aqueous tin(II) nitrate (Sn(NO₃)₂), a double-replacement reaction takes place. The balanced chemical equation for the reaction is,
Na₂CO₃(aq) + Sn(NO₃)₂(aq) → SnCO₃(s) + 2NaNO₃(aq)
In this reaction, the sodium ion and nitrate ion from the sodium carbonate and tin(II) ion and nitrate ion from the tin(II) nitrate switch places to form solid tin(II) carbonate and aqueous sodium nitrate.
To know more about the double-replacement reaction, here
brainly.com/question/29307794
#SPJ4
What is the mass of 4.2kg gold when it's transferred to a planet having twice the gravity of earth
Answers
Answer:
The only relationship is that the larger the mass of an object the more gravitational force it has. So... something that has more mass has more gravity. The mass of 4.2kg of gold when transferred to this new planet is still 4.2kg. However, the WEIGHT (not mass) is now doubled to 8.4kg. Mass is constant it is how much matter something is made up of. Weight depends on the gravitational force. Moreover, I would look at other answers too (I'm not entirely confident of saying that the weight is 8.4kg since I don't have any gravitational equations in front of me it just sounds logical).
Explanation:
What are the two main components of the peripheral nervous system?
A.
the sympathetic nervous system and spinal cord
B.
the sensory and motor neurons
C.
the brain and spinal cord
D.
the parasympathetic nervous system and senses
Answers
Answer: i believe it would be A
Answer:
a and d
Explanation:
To prepare 800mL of 1M phosphate buffer of pH 7.2 (PKa=7.1), by using salt = K2HPO4 and acid = KH2PO4, the quantity of salt required is
Answers
Answer:
78.04g of 0.448 moles must be added
Explanation:
Using H-H equation we can find the pH of the buffer:
pH = pKa + log [A⁻] / [HA]
Where pH is the pH of the buffer = 7.2
pKa = 7.1
[A⁻] = [K₂HPO₄]
[HA] = [KH₂PO₄]
Replacing:
7.2 = 7.1 + log [K₂HPO₄] / [KH₂PO₄]
0.1 = log [K₂HPO₄] / [KH₂PO₄]
1.2589 = [K₂HPO₄] / [KH₂PO₄] (1)
And as the concentration of the buffer is:
1M = [K₂HPO₄] + [KH₂PO₄] (2)
Replacing (2) in (1):
1.2589 = 1M - [KH₂PO₄] / [KH₂PO₄]
1.2589 [KH₂PO₄] = 1M - [KH₂PO₄]
2.2589 [KH₂PO₄] = 1M
[KH₂PO₄] = 0.44M
And [K₂HPO₄] = 0.56M
In 800mL = 0.8L:
0.8L * (0.56mol / L) = 0.448 moles K₂HPO₄. The mass is -Molar mass K₂HPO₄: 174.2g/mol-:
0.448 moles * (174.2g / mol) =
78.04g of 0.448 moles must be addedPleasee help me :(
If a solution of aspirin has a [H3O+] = 1.7 x10 -3 M, what is the pH of the solution?
Answers
Answer:
2.77Explanation:
pH is defined as the negative logarithm of the hydrogen ion concentration of a substance.
In order to find the pH we use the formula;
\( \bold{pH = -log([{H_3O}^{+}])} \)
From the question
\( [{H_3O}^{+}]\) = \( {1.7 \times 10}^{-3} \: M \)
We have
\( pH = -log({1.7 \times 10}^{-3}) \\ = 2.769555 \)
We have the final answer as
pH = 2.77