Is this an ionic substance a covalent substance or a metal?
Answers
In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals.
As a general rule of thumb, compounds that contain a metal binding with both a non-metallic or a semi-metallic will show ionic bonding. Compounds that are composed of best non-metals or semi-metals with non-metals will show covalent bonding and may be categorised as molecular compounds.
those molecules that encompass charged ions with opposite prices are referred to as IONIC. these ionic compounds are generally solids with excessive melting points and conduct electric contemporary. Ionic compounds are typically formed from metal and a non-metal elements.
Covalent bonding normally happens between nonmetals. Covalent bonding is the sort of bond that holds together the atoms inside a polyatomic ion. It takes electrons to make a covalent bond, one from every bonding atom.
To know more about bonding click here
https://brainly.com/question/819068
#SPJ4
Related Questions
How do populations survive when the environment
changes?
Answers
Answer:
adaptation?
Explanation:
the amount of fluorine in a metal fluoride is 14.96%. 2 Chromium are connected to the metal when metal chromate is formed. What is the relative atomic mass of the metal
Answers
Answer:
The relative atomic mass of the metal is 207.2 u
Explanation:
Metal chromate
Given that;
1) The mass of fluorine is 14.96% of the metal fluoride
2) 2 Chromium are connected to the metal when the metal chromate, CrO²⁻, is formed
We have;
Number of ions available in the metal = Cr₂O₇²⁻ = +2 ionic
Molar mass of fluorine = 18.998 g/mole
Ionization of fluorine = -1
Number of moles of fluorine required per metal +2 ion= 2 moles
3) Number of moles of fluorine per mole of compound of the metal fluoride = 2 × moles
Mass of fluorine per mole of compound = 2 × 18.998 = 37.996 grams
Percentage by mass of fluorine = 14.96%
Fluoride
Let the mass of the compound = X
Therefore;
14.96% of X = 37.996 grams
X = 37.996/(0.1496) = 253.984 grams
Therefore the mass of the metal in the compound = 253.984 - 37.996 = Molar mass 215.99 grams
Given that the metal forms a chromate with 2 chromium atoms and a mass of 215.99 grams, the likely candidate is lead, Pb with a molar mass of 207.2 grams and a chromate of Pb(CrO₄)₂.
The fluoride, lead fluoride, F₂
The relative atomic mass of lead is 207.2 u
Fatty acid groups are referred to as ________ groups.
A) Acetyl
B) Acyl
C) Prenyl
D) Isoprenoid
E) Isopentenyl
Answers
Fatty acid groups are referred to as B) acyl groups.
Fatty acids are organic compounds that consist of a long hydrocarbon chain with a carboxyl group (-COOH) at one end. The hydrocarbon chain is composed of carbon and hydrogen atoms, and its length can vary. Fatty acids play essential roles in various biological processes and are major components of lipids, including triglycerides and phospholipids.
When a fatty acid is involved in chemical reactions or is attached to other molecules, it typically undergoes a process called activation, where it is converted into an acyl group. An acyl group is formed by replacing the -OH (hydroxyl) group of the carboxyl group with an -OR (alkoxy) group. The -OR group can be derived from various molecules, such as coenzyme A (CoA) or other acyl carrier proteins.
For example, when a fatty acid is activated for incorporation into a triglyceride molecule, it forms a triglyceride acyl group. Similarly, when a fatty acid is incorporated into a phospholipid molecule, it forms a phospholipid acyl group. The acyl group represents the hydrocarbon chain of the fatty acid, which may vary in length and saturation.
Therefore, the correct answer to the question is B) Acyl.
Learn more about Fatty acids https://brainly.com/question/27960389
#SPJ11
How many molecules are in 4. 62 moles of nitric acid.
Answers
Answer:
1 mol of nitric acid consists of 6.022 ✕ 10²³ molecules of nitric acid. The above discussion suggests that 4.62 moles of nitric acid have 27.822 ✕ 10²³ molecules.
Answer:
2.78 × 10²⁴ molecules
Moles and Avogadro's Constant:In chemistry, a mole (in mol, denoted by symbol 'n') is a unit of measurement, where 1 mole = 6.022×10²³ of anything. This unit of measurement is designed to make calculations more convenient when dealing with atoms and molecules, which are very small.
Avogadro's Constant is the name given for the value of 6.022×10²³.
If one mole is 6.022×10²³ molecules, then the number of molecules (N) in 'n' number of moles is n × Avogadro's Constant (\(N_A\).) Therefore:
\(\boxed{\huge \textsf{$ N = n\times N_A$}}\)
So if we have 4.62 moles of HNO₃ (nitric acid), then:
\(\Large \textsf{$ N(\rm HNO_3) = 4.62\times 6.022\times 10^{23}$}\)
\(\boxed{\boxed{\huge \textsf{$ \therefore N(\rm HNO_3) = 2.78 \times 10^{24}\, molecules$}}}\)
To learn more about Avogadro's Constant:
https://brainly.com/question/20888686
In each of the following radioactive decay processes, supply the missing particle.a. 60Co → 60Ni + ?b. 97Tc + ? → 97Moc. 99Tc → 99Ru + ?d. 239Pu → 235U + ?
Answers
a. 60Co → 60Ni + ?
In the decay of 60Co, a beta particle (β-) is emitted. Therefore, the missing particle is a beta particle (β-).
b. 97Tc + ? → 97Mo
In the decay of 97Tc, a beta particle (β-) is emitted. Therefore, the missing particle is a beta particle (β-).
c. 99Tc → 99Ru + ?
In the decay of 99Tc, a beta particle (β-) is emitted. Therefore, the missing particle is a beta particle (β-).
d. 239Pu → 235U + ?
In the decay of 239Pu, an alpha particle (α) is emitted. Therefore, the missing particle is an alpha particle (α).
In radioactive decay processes, different types of particles can be emitted, including alpha particles (α), beta particles (β- or β+), gamma rays (γ), and sometimes other particles like neutrons or protons. The specific type of particle emitted depends on the particular radioactive isotope and the type of decay involved.
To learn more about isotope click here:brainly.com/question/27475737
#SPJ11
Which of the following aspects of electromagnetic radiation best explains why electromagtic radion is both useful and harmful to humans? -can be described in terms of both wavelength and frequency; - travels at the speed of light; - can travel through a vaccum;- is energy and can interact with matter
Answers
The correct answer is option A.
Electromagnetic radiation is both useful and harmful to humans because they have both wavelength and frequency.
What are electromagnetic radiations?
A wave that consists of both electric and magnetic radiations is called an electromagnetic radiation.
These waves are also described in terms of having both wavelength and frequency.
In an empty space, these waves move with the speed of light and include gamma rays, radio waves, infrared light, X-rays, and ultraviolet rays.
Due to their high frequency, these waves are high energy waves and are used to inhibit the growth of unnecessary cells inside body but they can also cause damage to healthy cells and body tissues.
If you need to learn more about electromagnetic radiation click here:
https://brainly.com/question/1408043
#SPJ4
A chemist experiments on a molecule with the formula of c5h10o5. this compound is likely a(n) ________.
Answers
A chemist experiments on a molecule with the formula of c5h10o5. this compound is likely a(n) carbohydrates.
A molecule with the formula C₅H₁₀O₅ is carbohydrates.
What are Carbohydrates?
The carbohydrates are represented through the chemical system Cₙ(H₂O)ₓ.C denotes carbon within the components with a few water molecules. hence, carbohydrates mean hydrated carbon as it has some water molecules attached.The ratio of carbon, hydrogen and oxygen in carbohydrates is 1:2:1.Glucose is the maximum plentiful and simple carbohydrate.Monosaccharides and polysaccharides are the 2 kinds of carbohydrates.Carbohydrates are biomolecules.they also offer electricity to living things through numerous meals like bread, and rice, and so forth.therefore the molecular method C₅H₁₀O₅ represents carbohydrates.
To learn more about Carbohydrates, visit: https://brainly.com/question/14614055
#SPJ4
difference between intermediate and activated complex
Answers
Answer:
The activated complex is an unstable (unobserved) structure corresponding to an energy maximum in the reaction profile. An intermediate is the chemical structure present at the transition state. An activated complex can not be isolated or observed in a chemical reaction.
Explanation:
The particles in a solid are packed very closely together in a rigid, orderly
arrangement. They are held together by the attractive forces that act
between all particles of matter. Solids, therefore, have fixed volumes and
shapes. In the solid state, particles cannot break away from their fixed
position; they can only vibrate in place.
The particles that make up a liquid move more rapidly than those in a solid
do. This causes the particles in the liquid to overcome some of the
attractive forces between them, and the particles can slide freely around
each other.
The particles of a gas are very far apart and move rapidly compared to
particles in solids or liquids. At these distances, the attractive forces
between gas particles have a lesser effect than they have on particles in
liquids and solids. In general, the volume of a liquid or solid increases
greatly when it forms a gas. However, the density of the gaseous state of
most substances is approximately one-thousandth the density of the liquid
state.
Plasma is a gas in which the particles have so much energy that they
become electrically charged. Plasma is the most common state of matter in
the universe, with more than 99.99% of observable matter in the universe
being plasma. Most of the matter of the sun is plasma. Stars and lightning
are also examples of plasma. However, this state is not experienced in the
physical and chemical changes we encounter every day on Earth.
Answers
plasma can be produced artificially in a laboratory but only for a few nanosecond or minutes
Explanation:
in other to maintain plasma reactions you are going to need state of the art technology such as particle accelerators but even those cannot meet the full amounts of keeping plasma reactions stable for a long time the reason for that is because plasma reactions require a lot of energy
In which of the following aqueous solutions does the weak acid exhibit the highest percentage ionization?
A. 0.01 M HSO3â (Ka = 6.3 â10â8)
B. 0.01 M HF(Ka = 6.3 â 10â4)
C. 0.01 M H3BO3 (Ka = 5.4 â10â10)
D. 0.01 M HC2H3O2 (Ka = 1.8 â 10â5)
E. 0.01 M H2C2O4 (Ka = 5.8 â10â2)
Answers
The weak acid will exhibit the highest percentage ionization in the solution with the highest Ka value.
To determine the highest percentage ionization, we need to compare the acid dissociation constant (Ka) values of the given weak acids. A higher Ka value indicates a higher degree of ionization in the solution. Here are the Ka values for each option:
A. HSO3⁻ (Ka = 6.3 × 10^(-8))
B. HF (Ka = 6.3 × 10^(-4))
C. H3BO3 (Ka = 5.4 × 10^(-10))
D. HC2H3O2 (Ka = 1.8 × 10^(-5))
E. H2C2O4 (Ka = 5.8 × 10^(-2))
From the given Ka values, we can see that HF (B) has the highest Ka value, which means it has the highest percentage ionization among the given aqueous solutions of weak acids.
To know more about ionization visit:
https://brainly.com/question/1602374
#SPJ11
You are tasked with making a series of Furosemide calibration standards for analysis by HPLC with fluorescence detection.
(a) Given Furosemide has a molecular weight of 330.7 g/mol, what weight of furosemide would you need to make a stock solution of 0.001 M (10-3 M) concentration ?
(b) How would you then make up a series of standards from this stock solution, of concentrations 10-4, 10-5, 10-6, 10-7 and 10-8 M Furosemide?
Answers
(b) To make up a series of standards from the stock solution, you would first dilute the stock solution with water to make a working solution of 10-4 M concentration. You would then take 1 mL of the working solution and dilute it with water to make a 10-5 M concentration standard, and so on, until you have standards of 10-4, 10-5, 10-6, 10-7, and 10-8 M concentrations.
How do you find the number of neutrons for any atom?
Answers
the theory that the collapse of a massive star's iron core produces neutrinos was supported by
Answers
The theory that the collapse of a massive star's iron core produces neutrinos was supported by the detection of a burst of neutrinos from the direction of Supernova 1987A in the Large Magellanic Cloud.
This burst of neutrinos was detected by several neutrino observatories around the world, including Kamiokande II in Japan and IMB in the United States, which detected a total of 24 neutrino events.
The time of arrival and energy spectrum of these neutrinos were consistent with the predictions of the theory of core-collapse supernovae, providing strong evidence for the idea that massive stars end their lives in a violent explosion, accompanied by the emission of large numbers of neutrinos.
To know more about neutrinos , refer here :
https://brainly.com/question/27870530#
#SPJ11
Volcanic eruptions can be very dangerous for nearby human and animal populations. One danger is that lava and ash may bury habitats. What is another danger during a volcanic eruption?
A.
Lava or hot debris may start fires.
B.
Lava flows may cause tsunamis.
C.
Volcanic eruptions always cause flooding.
D.
Volcanic eruptions always cause high winds.
Answers
Answer:
B. Lava flows may cause tsunamis.
Which of the circled protons in the molecules below would absorb furthest downfield in NMR?
Answers
To determine which of the circled protons in the molecules would absorb furthest downfield in NMR (nuclear magnetic resonance), we need to consider the factors that influence chemical shift values.
The chemical shift is a measure of the proton's electron density and its surrounding environment. Protons experience different shielding effects based on the electron distribution around them. Electron-withdrawing groups or deshielding environments cause protons to be less shielded and absorb further downfield (at higher ppm values), while electron-donating groups or shielding environments cause protons to be more shielded and absorb further upfield (at lower ppm values).
Without the provided images of the molecules and the specific functional groups or substituents attached to the circled protons, it is difficult to provide a precise answer. However, I can offer some general guidelines:
Electronegative atoms: Protons near electronegative atoms such as oxygen, nitrogen, or halogens tend to absorb further downfield due to their deshielding effect.
Conjugated systems: Protons in conjugated systems or aromatic rings can also absorb further downfield due to the delocalization of electrons, which reduces their shielding.
Electron-withdrawing groups: If the circled protons are adjacent to electron-withdrawing groups like carbonyl (C=O) or nitro (NO2), they are likely to absorb further downfield.
Ring currents: Protons in close proximity to aromatic rings or cyclic structures may experience ring currents that can affect their chemical shift values.
Remember that these guidelines are general, and the actual positions of absorption depend on the specific molecular structures and environments of the circled protons.
To know more about NMR spectra click this link -
brainly.com/question/9784910
#SPJ11
KINDLY PARAPHRASE THE FOLLOWING PARAGRAPHS:
-------------------------------------------------------------------------------------------------------------------------
Growth in Distribution Spaces
An essential part of the e-commerce business is its supply chain. Figuring out the logistics for packaging and shipping goods to customers includes warehousing, and that’s where commercial real estate comes into play. As e-commerce has grown, we have seen significant growth in the leasing and sale of distribution centers and warehouse spaces.
E-commerce giants look for spaces near large cities like Houston while still having enough space for large buildings. There is a lot of potential and growth in the Houston suburbs such as Katy, Brookshire & Waller. We are seeing more distribution centers popping up in these areas.
Smaller Retail Spaces
As retail has shifted to online, we have seen businesses struggling to keep physical spaces open over the past few years. While e-commerce is booming, some brick-and-mortar spaces are having to close or downsize.
There are certain markets, like groceries, that will always require a physical location, but there is a trend for smaller retail spaces across the market. Smaller spaces mean less inventory in-store, and this consequently encourages a combination of online and in-store shopping. Hybrid shopping especially increased in popularity during the Covid-19 lockdown.
Merging online shopping with curbside or in-store pick-up offered that element of convenience and a safe way to shop during the pandemic, and even as restrictions ease, people will still seek the ease of this approach. However, even though convenience is what mainly drives e-commerce, we don’t expect to see in-store experiences disappear altogether.
Increased Technology in Retail
Since many prefer shopping online, working to translate the benefits of technology to physical spaces has been important in keeping up with trends. Integrating technology into retail spaces will be essential for future leasing and selling opportunities in the market. Implementing tools such as apps can create unique and convenient shopping experiences and can help businesses gather data that is essential for tracking traffic and learning more about the customer.
These tools can also help drive customers to the retail location with special offers or in-store pickup options. Large lifestyle shopping centers have shown to be among the most proactive in blending technology with consumer experiences.
Overall, e-commerce has a major impact on the commercial real estate business, from the industrial real estate benefit from its growth to seeing space buying and leasing becoming a smaller part of retail operations. In 2020 alone, e-commerce accounted for 14 percent of all sales, but it is inevitable that e-commerce will continue to grow as it has for the last decade. Commercial real estate is a reflection of society and its habits and we will continue to see it mirrored as changes in technology and retail emerge.
---------------------------------------------------------------------------------
Answers
The impact of e-commerce on commercial real estate is significant. E-commerce sales have grown steadily, accounting for a considerable portion of overall sales.
The growth of e-commerce has fueled the demand for distribution spaces, specifically distribution centers and warehouses, which play a crucial role in the supply chain and logistics of packaging and shipping goods to customers. These spaces are sought after by e-commerce giants, who prefer locations near large cities while still providing ample room for large buildings. Suburban areas, such as Katy, Brookshire, and Waller near Houston, are experiencing significant growth in the establishment of distribution centers.
On the other hand, the rise of online shopping has posed challenges for brick-and-mortar retailers. Many physical retail spaces have struggled to remain open or have had to downsize. As a result, there is a trend towards smaller retail spaces, which require less inventory in-store. This trend encourages a combination of online and in-store shopping, known as hybrid shopping. The Covid-19 pandemic further accelerated this trend as consumers sought the convenience and safety of online shopping with options like curbside or in-store pick-up. Even as restrictions ease, this approach is expected to remain popular.
To adapt to the changing retail landscape, integrating technology into physical retail spaces has become crucial. Technology tools, such as mobile apps, can enhance the shopping experience, offer special promotions, and provide valuable data on customer behavior. Retailers, especially large lifestyle shopping centers, have been proactive in blending technology with consumer experiences to stay relevant and attract customers.
Learn more about Covid-19 pandemic here:
https://brainly.com/question/30975256
#SPJ11
If you know your speed and how long you have been traveling, then you can calculate your dsitance with the formula
Answers
Speed is calculated as follows: speed = distance x time. Knowing the units for distance and time is necessary to determine the units for speed. Then distance = speed/time.
What is distance?The distance traveled must be divided by the amount of time spent traveling in order to calculate speed. Divide the distance by the speed to calculate the time. Add the speed and the time together to get the distance. These equations can be seen as s=d/t when speed, distance, and time are all given.
The distance formula, d = st, or distance equals speed times time, can be used to calculate distance. Speed and rate are comparable because they both denote a certain amount of travel per unit of time, such as miles per hour or kilometers per hour. If speed s is equal to rate r, then r = s = d/t.
To know more about distance visit:
https://brainly.com/question/11567236
#SPJ1
Describe the best method for separating a mixture
of sand, salt, and steel shavings by placing the
steps below in the correct order.
Heat the mixture to evaporate the water.
Pour the mixture through a filter to separate
the sand
Pass a magnet over the mixture to remove
the steel.
Add water to the sand/salt mixture and stir to
dissolve the salt.
Answers
Answer:
Filtration is a method for separating an insoluble solid from a liquid. When a mixture of sand and water is filtered: the sand stays behind in the filter paper (it becomes the residue ) the water passes through the filter paper (it becomes the filtrate )
Explanation:
Answer:
Describe the best method for separating a mixture of sand, salt, and steel shavings by placing the steps below in the correct order.
⇒ 4 Heat the mixture to evaporate the water.
⇒ 3 Pour the mixture through a filter to separate the sand.
⇒ 1 Pass a magnet over the mixture to remove the steel.
⇒ 2 Add water to the sand/salt mixture and stir to dissolve the salt.
Superfine 40 gauge copper wire has a diameter of only 0.080 mm and weighs only 44.5 g/km. Suppose it’s full of 40 gauge wire weighs 471. g Less after some wire is pulled off to wind a magnet. How could you calculate how much wire is used?Set the matter. But don’t do any of it. Just leave your answer as a math expression. Also be sure your answer includes all the correct unit symbols.Length of wire = ?

Answers
- To calculate the length of wire we can use a mathematical Rule of Three:
\(\text{length of wire=}\frac{wire\text{ weighs. 1km}}{44.5g}\)- So, in this case the length would be:
\(\begin{gathered} \text{length of wire=}\frac{\text{471g.1km}}{44.5g} \\ \text{length of wire=10.58km} \end{gathered}\)We can write the math expression as:
\(L=\frac{w.1\operatorname{km}}{44.5g}\)Here, L is the length of wire and w is the wire weighs.
how does atmospheric carbon dioxide affect global temperature?
Answers
Answer:
Carbon dioxide is a greenhouse gas a gas that absorbs and radiates heat.
Explanation:
an aluminum atom has a mass of and a small airplane has a mass of . use this information to answer the questions below. be sure your answers have the correct number of significant digits. what is the mass of 1 mole of aluminum atoms? round your answer to 3 significant digits. how many moles of aluminum atoms have a mass equal to the mass of a small airplane? round your answer to 3 significant digits.
Answers
(a) The mass of 1 mole of aluminum atoms is 26.982 g.
(b) The number of moles of aluminum atoms that have a mass equal to the mass of a small airplane is 185,250 mol.
(a) To calculate the mass of 1 mole of aluminum atoms, we use the molar mass of aluminum, which is 26.982 g/mol (rounded to 3 significant digits). The mass of 1 mole of aluminum atoms is therefore:
1 mole of Al atoms x 26.982 g/mol = 26.982 g
(b) To find how many moles of aluminum atoms have a mass equal to the mass of a small airplane, we need to convert the mass of the airplane from kilograms to grams:
5000 kg x 1000 g/kg = 5,000,000 g
Next, we need to divide the mass of the airplane by the mass of 1 mole of aluminum atoms to get the number of moles of aluminum atoms:
number of moles = mass of airplane / molar mass of aluminum
5,000,000 g / 26.98 g/mol = 185,250 mol
Therefore, 185,250 moles of aluminum atoms have a mass equal to the mass of a small airplane.
For more question on mass click on
https://brainly.com/question/837939
#SPJ11
CORRECT QUESTION WOULD BE
An aluminum atom has a mass of 4.48 * 10^23 g and a small airplane has a mass of 5000 kg. Use this information to answer the questions below. Be sure your answers have the correct number of significant digits.
(a) What is the mass of 1 mole of aluminum atoms?
(b) How many moles of aluminum atoms have a mass equal to the mass of a small airplane?
11 grams of carbon dioxide, CO2, is dissolved in 1000 mL of solution. Determine the molarity (M).
Answers
Answer: The molarity is 0.25 M
Explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
\(Molarity=\frac{n\times 1000}{V_s}\)
where,
n = moles of solute
\(V_s\) = volume of solution in ml
moles of \(CO_2\) = \(\frac{\text {given mass}}{\text {Molar mass}}=\frac{11g}{44g/mol}=0.25mol\)
Now put all the given values in the formula of molality, we get
\(Molarity=\frac{0.25\times 1000}{1000ml}\)
\(Molarity=0.25M\)
Therefore, the molarity is 0.25 M
You are a nurse giving a patient 0.75 cc per minute of a drug cocktail intravenously . A cc is 1ml of a solution . If the patient needs thr iv for 2 hours how many liters of this solution will you need to prepare
Answers
Answer:
90 mL
0.09 Liters
Explanation:
Givens
1 cc of solution = 1 mL
0.75 cc / min needed to go in intravenously.
2 hours needed.
? Liters
Solution
1 hour = 60 minutes
1 hours = 2 * 60 = 120 minutes
rate = 0.75 cc/minute
Liters = rate * time
Liters = 0.75 cc * 120 minutes
Liters = 90 cc
That's not the answer.
1 Liter has 1000 mL or ccs
x Liter has 90 mL
Equation
1/x = 1000 / 90
Solution
Cross multiply
1000x = 90*1 Divide by 1000
x = 90/1000
x = 0.09 Liters.
Complete the following radioactive decay problem. Please help

Answers
Answer:
230 90Th
Explanation:
A careful observation of the equation given in question shows that 234 92U is undergoing alpha decay. This means that the resulting daughter nuclei will have a decrease of 4 in the mass number and a decrease of 2 in the atomic number.
Please see attached photo for further details.
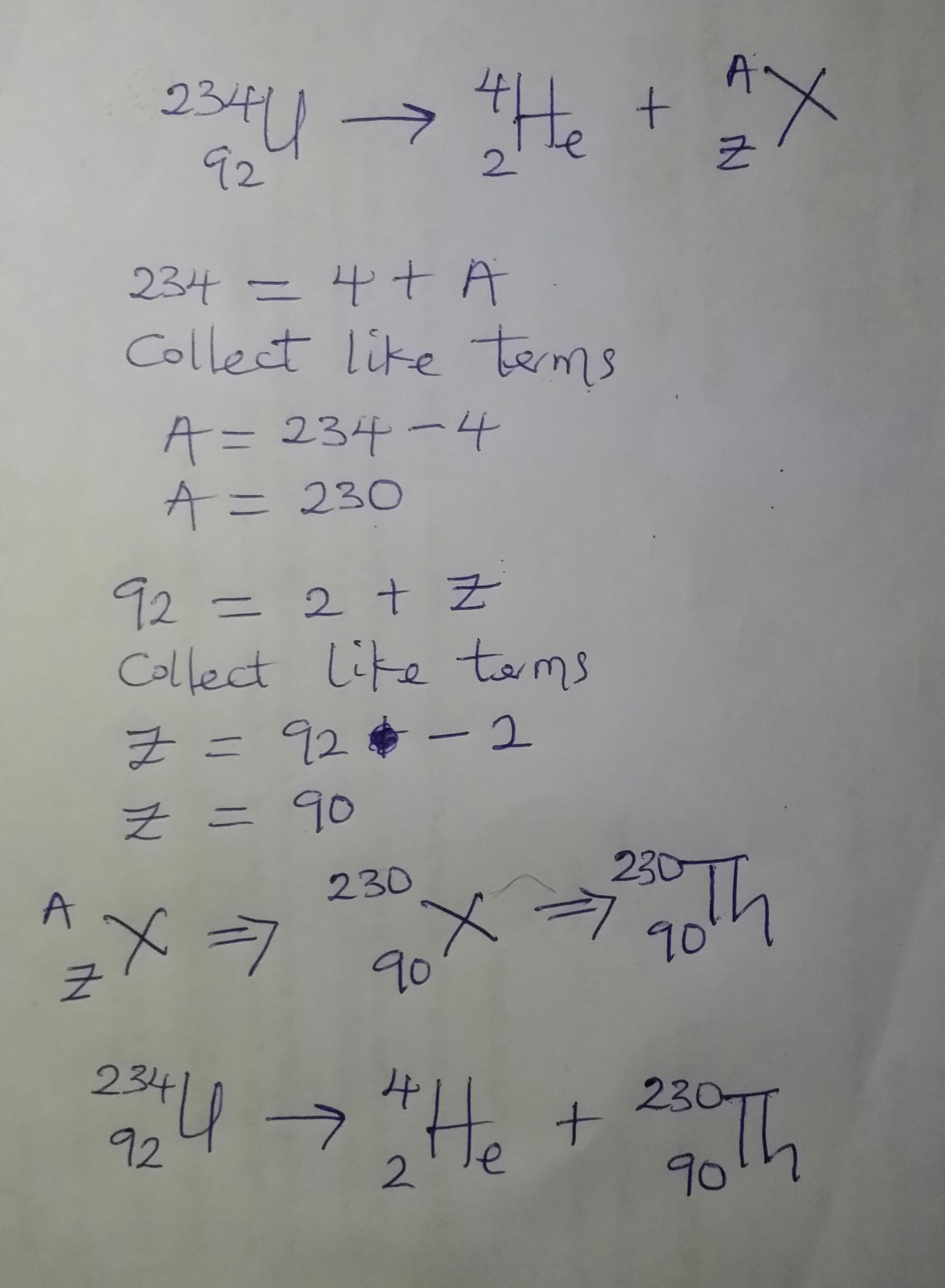
the photo has the question; please help asap ! i’m giving brainliest—i also have more chemistry, honestly i’m about to post a whole bunch of questions

Answers
Answer:
The process is to divide the molecules by Avogadro's number (6.02 x 10^23) to get the moles. And since it's specified at STP (standard temperature and pressure) we know that the molar volume would be 22.4L. So we can just multiply the moles by the molar volume in order to get the volume of H2S.
(2.18 x 10^24) / (6.02 x 10^23) = 3.62 moles (rounded)
Now just multiply the moles by the molar volume of STP, 22.4.
3.62 x 22.4 = 81 Liters (rounded) of H2S
please help, ive been putting 3 for the coefficient and 14 for the exponent but, it keeps saying it’s wrong. any help is appreciated.

Answers
Answer:
3 × 10¯¹⁰
Explanation:
9×10² ÷ 3×10¹²
The above expression can be evaluated as follow:
9×10² ÷ 3×10¹²
Recall:
3² = 9
3¹ = 3
Therefore,
9×10² ÷ 3×10¹² = 3²×10² ÷ 3¹×10¹²
Recall:
y^m ÷ y^n = y^(m – n)
Therefore,
3²×10² ÷ 3×10¹² = 3²¯¹ × 10²¯¹²
= 3¹ × 10¯¹⁰
Recall:
y¹ = y
Therefore,
3¹ × 10¯¹⁰ = 3 × 10¯¹⁰
Therefore,
9×10² ÷ 3×10¹² = 3 × 10¯¹⁰
Which of the following is the smallest level of organization?
Select one:
tissue
organ
cell
organ system?
Answers
Answer:
Cell
Explanation:
Cells are the smallest level
what is the rate of formation of oxygen gas in an experiment where 0.08 mol of n2o5 is consumed in a 4.0l
Answers
The rate of formation of oxygen gas in an experiment where 0.08 mol of N2O5 is consumed in a 4.0L reaction vessel is 0.02 mol/L/s.
To calculate the rate of formation of oxygen gas, use the balanced equation:
N2O5(g) → 2NO2(g) + 1/2O2(g)
The stoichiometry of the reaction implies that 1 mole of N2O5 produces 1/2 mole of O2 gas. So, if 0.08 mole of N2O5 is used up, 1/2 x 0.08 mole = 0.04 mole of O2 gas will be formed.
The volume of the reaction vessel is given as 4.0L. Therefore, the concentration of O2 gas is 0.04 mole/4.0 L = 0.01 mol/L
The experiment's rate is given as the rate of formation of O2 gas, which is the rate of its appearance. Since the stoichiometry of the reaction shows that 1 mole of N2O5 produces 1/2 mole of O2 gas, then the rate of formation of O2 gas equals half the rate of consumption of N2O5 gas.
Therefore, Rate of formation of O2 gas = 1/2 x Rate of consumption of N2O5 gas
We can calculate the rate of consumption of N2O5 gas as follows:
Let's say that the initial concentration of N2O5 gas is [N2O5]0, and that after some time t, the concentration is [N2O5]t.
Then, the rate of consumption of N2O5 gas, RC, is given by:
RC = -Δ[N2O5]/Δt = ([N2O5]0 - [N2O5]t)/t
where the negative sign indicates that the concentration of N2O5 is decreasing. We can rearrange this equation to solve for Δ[N2O5]/Δt, which is the average rate of consumption of N2O5 gas during the time interval Δt:Δ[N2O5]/Δt = ([N2O5]t - [N2O5]0)/t
The experiment's rate of consumption of N2O5 gas is not given in the question. However, we can calculate it if we know the initial concentration of N2O5 gas, [N2O5]0, and the concentration after some time t, [N2O5]t.
For example, if [N2O5]0 = 0.1 mol/L and [N2O5]t = 0.05 mol/L after 5 seconds, then:
RC = ([N2O5]0 - [N2O5]t)/t = (0.1 - 0.05)/5 = 0.01 mol/L/s
Therefore, the rate of formation of O2 gas, which is half the rate of consumption of N2O5 gas, is:
Rate of formation of O2 gas = 1/2 x 0.01 mol/L/s = 0.005 mol/L/s = 0.02 mol/L/s (using the previously calculated value of the rate of consumption of N2O5 gas).
Hence, the rate of formation of oxygen gas in an experiment where 0.08 mol of N2O5 is consumed in a 4.0L reaction vessel is 0.02 mol/L/s.
To know more about rate of formation, refer here:
https://brainly.com/question/2496500#
#SPJ11
Can someone help me

Answers
Answer:
c. use a screen to filter out the sand
Explanation:
If there are tiny holes in the filter, the sand can go through those and the rock/artifacts stay in the pan.
Complete and balance the following equation
As2O3(s)+NOâ3(aq)âH3AsO4(aq)+N2O3(aq) (acidic solution)
Answers
The balanced equation for the given reaction is: \(As_2O_3(s) + 4NO_3^-(aq) + 4H^+(aq) \rightarrow 2H_3AsO_4(aq) + 2N_2O_3(aq) + 6H_2O(I) \)
To balance the equation, we first need to make sure that the number of atoms of each element is the same on both sides. In this case, we have 2 As atoms, 6 O atoms, 4 N atoms, and 4 H atoms on the reactant side. On the product side, we have 2 As atoms, 6 O atoms, 4 N atoms, and 6 H atoms.
To balance the equation, we can start by adding 4 H+ ions to the reactant side to balance the H atoms. This gives us:
\(As_2O_3(s) + 4NO_3^-(aq) + 4H^+(aq) \rightarrow H_3AsO_4(aq) + N_2O_3(aq)\)
Next, we need to balance the charge by adding electrons to the reactant side. We can do this by adding 12 electrons to the left side. This gives us:
\(As_2O_3(s) + 4NO_3^-(aq) + 4H^+(aq) \rightarrow H_3AsO_4(aq) + N_2O_3(aq)\)
Finally, we balance the equation by adding water molecules to the product side to balance the oxygen atoms. We need to add 6 \(H_2O\) molecules to the right side. This gives us the balanced equation:
\(As_2O_3(s) + 4NO_3^-(aq) + 4H^+(aq) + 12e^- \rightarrow 2H_3AsO_4(aq) + 2N_2O_3(aq) + 6H_2O(l)\)
Therefore, the balanced equation for the given reaction is \(As_2O_3(s) + 4NO_3^-(aq) + 4H^+(aq) \rightarrow 2H_3AsO_4(aq) + 2N_2O_3(aq) + 6H_2O(I) \) in an acidic solution.
Learn more about solution :
https://brainly.com/question/30665317
#SPJ11