Mixtures A chemist has three acid solutions at various concentrations. The first is 10% acid, the second is 20%, and the third is 40%. How many milliliters of each should she use to make 100 mL of 18% solution, if she has to use four times as much of the 10% solution as the 40% solution?
Answers
A system of equations refers to a set of multiple equations that are to be solved simultaneously, where the solutions should satisfy all the equations in the system.
Let's assume the chemist needs to use x milliliters of the 40% acid solution. According to the given information, she would need to use four times as much of the 10% solution, which would be 4x milliliters.
To determine the amount of the 20% solution, we can subtract the sum of the amounts used for the 10% and 40% solutions from the total desired volume of 100 mL. So, the amount of the 20% solution would be 100 mL - (4x + x) mL = 100 mL - 5x mL.
Now, we can calculate the total amount of acid in the mixture:
Amount of acid from the 10% solution: (4x mL) × 0.10 = 0.4x mL
Amount of acid from the 20% solution: [(100 mL - 5x mL) mL] × 0.20 = 20 mL - x mL
Amount of acid from the 40% solution: (x mL) × 0.40 = 0.4x mL
The total amount of acid in the mixture is the sum of the acid amounts from each solution:
0.4x mL + (20 mL - x mL) + 0.4x mL = 18 mL
Simplifying the equation, we get:
20 mL + 0.4x mL - x mL + 0.4x mL = 18 mL
0.8x mL = -2 mL
x ≈ -2.5 mL
Since we cannot have a negative volume, this implies that there is no solution that satisfies the given conditions.
To make 100 mL of an 18% acid solution, the chemist should use 40 mL of the 10% acid solution, 10 mL of the 20% acid solution, and 5 mL of the 40% acid solution. By mixing these quantities, she would obtain the desired concentration.
To know more about system of equations click on below link:
brainly.com/question/21620502
#SPJ11
Related Questions
he primary compound responsible for acidity in unripe grapes.
Answers
The primary compound responsible for acidity in unripe grapes is tartaric acid.
Tartaric acid is a dicarboxylic acid that is naturally found in many fruits, including grapes. It contributes to the tart, sour taste of unripe grapes and is an important factor in determining the overall flavour of the grapes.
Tartaric acid is synthesized in the grape berry during the early stages of development and accumulates in the vacuoles of the grape cells. As the grapes ripen, the tartaric acid content decreases and the grapes become sweeter.
The concentration of tartaric acid in grapes can vary depending on several factors, including grape variety, climate, soil type, and vineyard management practices. In general, grapes grown in cooler climates or at higher elevations tend to have higher levels of tartaric acid, while grapes grown in warmer climates or in sandy soils tend to have lower levels.
Winemakers pay close attention to the levels of tartaric acid in grapes because it can have a significant impact on the resulting wine. High levels of tartaric acid can result in a wine that is too tart or sour, while low levels can result in a wine that is lacking in acidity and flavour. Therefore, winemakers may adjust the levels of tartaric acid in the wine by adding tartaric acid or performing processes such as malolactic fermentation, which converts malic acid (another acid found in grapes) into lactic acid, resulting in a smoother, less tart wine.
To learn more about tartaric acid, refer:-
https://brainly.com/question/24244696
#SPJ11
How many grams are there in 4 moles of potassium chloride?.
Answers
To calculate the number of grams in 4 moles of potassium chloride (KCl), the atomic masses of potassium and chlorine have to be found. It is then added to obtain the molar mass of KCl.
Finally, multiply the molar mass of KCl by the number of moles of KCl to determine the amount of KCl in grams. Potassium has a molar mass of 39.1 grams/mole, and chlorine has a molar mass of 35.5 grams/mole. Therefore, the molar mass of potassium chloride (KCl) is calculated as follows:Molar mass of KCl = 39.1 g/mol + 35.5 g/molMolar mass of KCl = 74.6 g/molTo compute the number of grams in 4 moles of KCl, the following formula is used:Number of grams = Number of moles × Molar massNumber of grams = 4 mol × 74.6 g/molNumber of grams = 298.4 gTherefore, 4 moles of potassium chloride (KCl) contain 298.4 grams of KCl. Answer: There are 298.4 grams in 4 moles of potassium chloride.
For more information on potassium chloride visit:
brainly.com/question/31104976
#SPJ11
The water cycle. Solar energy and wind are responsible for global weather patterns.Look at the diagram of the water cycle. What process is responsible for adding moisture to the atmosphere that could eventually fuel a hurricane.
PLLLLSSSS HELLLLLLPPPPPPPP
HELLLLLLPPPPPPP MEEEE PLSSSS
A. Evaporation of ocean water
B. Precipitation due to condensation
C. Movement of clouds through the troposphere

Answers
A. The process responsible for adding moisture to the atmosphere that could eventually fuel a hurricane is evaporation of ocean water.
What is water cycle?The water cycle describes how water evaporates from the surface of the earth, rises into the atmosphere, cools and condenses into rain or snow in clouds.
The water cycle involves the following;
Evaporation - this is the process through which water from seas, river, oceans, etc escape into the atmosphere.Condensation: this process occurs when the hot water vapor comes in contact with a cooler surface of the atmosphere, lose heat and condense into water droplets.Precipitation - this is the process through which the condensed water droplets saturates the atmosphere and begins to fall as rain water.Thus, the process responsible for adding moisture to the atmosphere that could eventually fuel a hurricane is evaporation of ocean water.
Learn more about water cycle here: https://brainly.com/question/25796102
#SPJ1
1,3-Butadiene is a conjugated diene with the chemical formula C4H6true or false
Answers
It is true that 1,3-butadiene has the chemical formula C4H6 and is a conjugated diene. A popular monomer in the creation of synthetic rubber, polymers, and resins, it is a colourless, flammable gas.
A conjugated diene with the chemical formula C4H6 is 1,3-butadiene. A popular monomer in the creation of synthetic rubber, polymers, and resins, it is a colourless, flammable gas. Because of the butadiene's conjugated double bond system, pi-electrons are delocalized over the whole molecule, giving it special characteristics including high reactivity, a low boiling point, and a propensity for addition reactions. Butadiene is a crucial industrial chemical that is created by the catalytic dehydrogenation of butene or butane. It finds usage in a variety of petrochemical, polymer, and automotive applications.
learn more about chemical formula here:
https://brainly.com/question/29031056
#SPJ4
Within a group, does the radii of atoms increase or decrease as the atomic
number increases? Explain.
Answers
Answer:increase
Explanation: the more electrons there are the bigger the electron field is and the bigger the atomic radii is
How many moles are in 1.23 x 1024 atoms of Calcium (Ca)?
Answers
The amount of substance of a system which contains as many elementary entities is called as mole. 2.05 moles are in 1.23 x 1024 atoms of Calcium (Ca).
What is mole ?The term mole is defined as the amount of substance of a system which contains as many elementary entities.The mole is called as containing exactly 6.023 × 10 ²³ elementary unit.
The mole is a convenient unit to use because of the great number of atoms, molecules. It is represented by symbol "mol".
1 mole of calcium = 6.023 × 10 ²³
1 mole ⇒ 6.023 × 10 ²³
X ⇒ 1.23 x 10²⁴
X = 12.3 x 10²³ / 6.023 × 10 ²³
= 2.05 moles
Thus, 2.05 moles are in 1.23 x 1024 atoms of Calcium (Ca).
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
Which accounts for the highest water withdrawals in the United States?(1 point)
Answers
Answer:
Agriculture accounts
Answer: Thermoelectric power
Explanation:
took the test!! good luck :)
An object has a mass of 183.5 g and a density of 14.8 g/cm³. Determine the volume of the objectin cm³.
Answers
First, let's remember the formula to calculate an object's density:
\(\begin{gathered} \rho=\text{ }\frac{m}{V} \\ \\ Being\text{ }\rho\text{ the density, m the mass, and V the volume.} \end{gathered}\)Then, we analyze what we have:
\(\begin{gathered} m\text{ = 183.5 g} \\ \rho=\text{ 14.8 g/cm}^3 \end{gathered}\)We need to determine the volume, so we transform our formula like this:
\(V=\text{ }\frac{m}{\rho}\)We replace our data:
\(V=\text{ }\frac{183.5\text{ g}}{14.8\text{ g/cm}^3}=\text{ 12.399 cm}^3\approx\text{ 12.4 cm}^3\)Then, the answer is that the volume equals 12.4 cm^3.
Hydrogen bonding between water molecules makes them tend to stick together. How does this affect the specific heat (SH) and heat of vaporization (HOV) of water
Answers
Answer:
The specific heat and heat of vaporization of water will be very high.
Explanation:
The strength of the hydrogen bonds and the attractions that they create between water molecules makes it much harder for water to increase in temperature.
This is because the bonds need to be broken before the water can vaporize (heat of vaporization) or increase in temperature (specific heat)
The Subatier Reactor is projected to produce 909 kg of water per year. Calculate the number of grams of H2 that needs to be collected each month to produce this amount of water?
Answers
Approximately 16,946 grams of H₂ needs to be collected each month to produce 909 kg of water per year in the Sabatier Reactor.
Steps
The balanced chemical equation for the electrolysis of water is:
2H₂O(l) → 2H₂(g) + O₂(g)
From the equation, we can see that 2 moles of water produce 2 moles of H₂ gas. The molar mass of water is 18.015 g/mol, and the molar mass of H₂ is 2.016 g/mol.
To calculate the number of grams of H₂ that needs to be collected each month to produce 909 kg of water per year, we can use the following steps:
Calculate the number of moles of water produced per year:
909 kg/year x (1000 g/kg) / 18.015 g/mol = 50,471.5 mol/year
Calculate the number of moles of H₂ produced per year:
50,471.5 mol/year x 2 mol H₂/mol H₂O = 100,943 mol/year
Calculate the number of moles of H₂ produced per month:
100,943 mol/year / 12 months = 8,412 mol/month
Convert the number of moles of H₂ to grams:
8,412 mol/month x 2.016 g/mol = 16,945.9 g/month
Therefore, approximately 16,946 grams of H₂ needs to be collected each month to produce 909 kg of water per year in the Sabatier Reactor.
learn more about moles here
https://brainly.com/question/15356425
#SPJ1
Is a backpack a good resource
for a rescue team?

Answers
how many grams of (s)-1-chloro-4-ethyl-2-methylhexane and triphenylphosphine would you need to create 4.15g of (s)-(4-ethyl-2- methylhexyl)triphenylphosphonium assuming an 81% yield for the reaction?
Answers
Approximately 1.52 g of (s)-1-chloro-4-ethyl-2-methylhexane and 2.12 g of triphenylphosphine would be required to get the required product.
How do you calculate the number of grams of reactants needed to get 81% yield of reaction?Molar mass of (s)-(4-ethyl-2-methylhexyl)triphenylphosphonium = (4 x molar mass of C) + (1 x molar mass of Cl) + (1 x molar mass of P) + (4 x molar mass of H) + (3 x molar mass of C6H5)
= (4 x 12.01 g/mol) + (1 x 35.45 g/mol) + (1 x 30.97 g/mol) + (4 x 1.01 g/mol) + (3 x 78.11 g/mol)
= 628.02 g/mol
Next, we can use stoichiometry to determine how much (s)-1-chloro-4-ethyl-2-methylhexane and triphenylphosphine are required to produce 4.15 g of (s)-(4-ethyl-2-methylhexyl)triphenylphosphonium. The balanced chemical equation for the reaction is:
(s)-1-chloro-4-ethyl-2-methylhexane + triphenylphosphine → (s)-(4-ethyl-2-methylhexyl)triphenylphosphonium chloride
From the balanced equation, we can see that one mole of (s)-1-chloro-4-ethyl-2-methylhexane reacts with one mole of triphenylphosphine to produce one mole of (s)-(4-ethyl-2-methylhexyl)triphenylphosphonium chloride. Therefore, we need to calculate the number of moles of (s)-(4-ethyl-2-methylhexyl)triphenylphosphonium chloride produced from 4.15 g, and then use stoichiometry to determine the amount of (s)-1-chloro-4-ethyl-2-methylhexane and triphenylphosphine required.
Number of moles of (s)-(4-ethyl-2-methylhexyl)triphenylphosphonium chloride = mass / molar mass
= 4.15 g / 628.02 g/mol
= 0.0066 mol
Since the yield is given as 81%, we need to adjust the amount of (s)-1-chloro-4-ethyl-2-methylhexane and triphenylphosphine accordingly:
Amount of (s)-1-chloro-4-ethyl-2-methylhexane = 0.0066 mol / 0.81
= 0.0081 mol
Amount of triphenylphosphine = 0.0066 mol / 0.81
= 0.0081 mol
Finally, we can convert the moles of each compound to grams using their respective molar masses:
Mass of (s)-1-chloro-4-ethyl-2-methylhexane = 0.0081 mol x 186.71 g/mol
= 1.52 g
Mass of triphenylphosphine = 0.0081 mol x 262.29 g/mol
= 2.12 g
Therefore, approximately 1.52 g of (s)-1-chloro-4-ethyl-2-methylhexane and 2.12 g of triphenylphosphine would be required to produce 4.15 g of (s)-(4-ethyl-2-methylhexyl)triphenylphosphonium assuming an 81% yield of reaction.
Learn more about yield of reaction here:
https://brainly.com/question/31082729
#SPJ1
Adipic acid is an organic compound composed of 49.31%C, 43.79%O, and the rest hydrogen. If the molar mass of adipic acid is 146.1 g/mol , what are the empirical and molecular formulas for adipic acid?
Answers
Considering the definition of empirical and molecular formula, the empirical formula is C₃H₅O₂ and the molecular formula is C₆H₁₀O₄.
Definition of empirical formulaThe empirical formula or minimal formula is one that indicates the type of atoms that make up a compound, the minimum ratio of the integer number of atoms and not necessarily the exact number of atoms in it.
Definition of molecular formulaThe molecular formula is the actual formula of the molecule and indicates the types of atoms and the number of each type involved in the formation of the molecule.
This formula can be obtained from the empirical or minimal formula, as long as the molecular mass of the compound is known. That is, divide the estimated molecular mass divided by the molecular mass of the empirical form. With the multiple obtained, we proceed to multiply the subscripts of the empirical formula, thus obtaining the molecular formula of the compound.
Empirical formula in this caseIn this case, you know:
Carbon (C): 49.31%Oxygen (O): 43.79%Hydrogen (H): 6.9%Assuming a 100 grams sample, the percentages match the grams in the sample. So you have:
Carbon (C): 49.31 gramsOxygen (O): 43.79 gramsHydrogen (H): 6.9 gramsThen it is possible to calculate the number of moles of each atom in the molecule, taking into account the corresponding molar mass:
Carbon (C): 49.31 grams÷ 12 g/mole= 4.109 molesOxygen (O): 43.79 grams÷ 16 g/mole= 2.74 molesHydrogen (H): 6.9 grams÷ 1 g/mole= 6.9 molesThe empirical formula must be expressed using whole number relationships, for this the numbers of moles are divided by the smallest result of those obtained. In this case:
Carbon (C): 4.109 moles÷ 2.74 moles= 1.5 Oxygen (O): 2.74 moles÷ 2.74 moles= 1Hydrogen (H): 6.9 moles÷ 2.74 moles= 2.5The empirical formula must be expressed using whole number relationships, so:
Carbon (C): 1.5× 2= 3Oxygen (O): 1× 2= 2Hydrogen (H): 2.5× 2= 5Therefore the C: H: O mole ratio is 3: 5: 2
Finally, the empirical formula is C₃H₅O₂.
Molecular formula in this caseThe molar mass of adipic acid is 146.1 g/mol and the molar mass of empirical formula is 69 g/mole.
Dividing the estimated molecular mass divided by the molecular mass of the empirical form, you get:
146.1 g/mol÷ 69 g/mol ≅ 2
This means that in the molecular formula there are 2 unit formulas, so it is necessary to multiply the number of all atoms by 2:
Molecular formula= 2×C₃H₅O₂
Molecular formula= C₆H₁₀O₄
The molecular formula is C₆H₁₀O₄.
Learn more about empirical and molecular formula:
brainly.com/question/26388921
brainly.com/question/14853529
brainly.com/question/13058832
#SPJ1
a saline solution was made by dissolving 1.69 g sodium chloride in 200 ml distilled water. what is the concentrtation of salt in this soution
Answers
a saline solution was made by dissolving 1.69 g sodium chloride in 200 ml distilled water,then the concentration of salt in the solution is 8.45 g/L.
To find the concentration of salt in the solution, we need to divide the amount of salt (in grams) by the volume of the solution (in liters).
First, we need to convert the volume from milliliters (mL) to liters (L):
200 mL = 0.2 L
Next, we can calculate the concentration of salt:
concentration = amount of salt / volume of solution
concentration = 1.69 g / 0.2 L
concentration = 8.45 g/L
Therefore, the concentration of salt in the solution is 8.45 g/L.
Learn more about concentration here:
https://brainly.com/question/10720472
#SPJ4
What is the area of a medium triangle?
Answers
Answer: Area of a Triangle Equals Base x Height / 3
Explanation: Hope this works
A double replacement reaction has two compounds as reactants.
True or false
Answers
Answer:
yes, A double replacement reaction has two compounds as reactants.
hence it's true.......
Answer:
it's true...
Explanation:
True or false, The four units that must always be used when using the ideal gas are 44.0 liters
Answers
Answer:
for volume only liters can be used
Explanation:
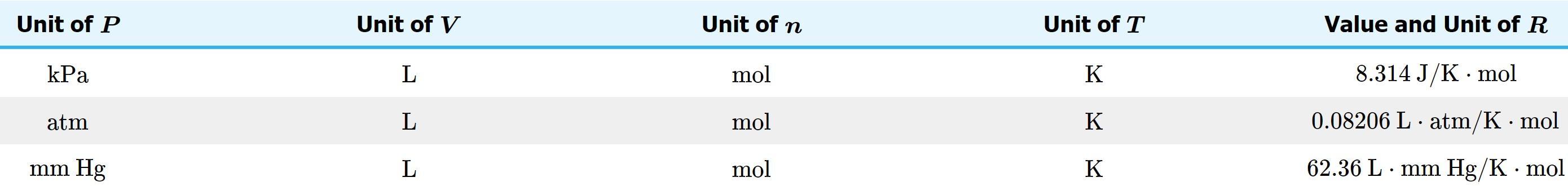
Calculate the atomic mass of oxygen if the three common isotopes of oxygen have masses of 15.995 amu (99.759% abundance), 16.995 amu (0.037 % abundance), and 17.999 amu (0.204 % abundance). (please show work) I will give brainliest answer !
Answers
Answer:
15.999 amu
Explanation:
From the question given above, the following data were:
Isotope A:
Mass of A 15.995 amu
Abundance (A%) = 99.759%
Isotope B:
Mass of B = 16.995 amu
Abundance (B%) = 0.037%
Isotope C:
Mass of C = 17.999 amu
Abundance (C%) = 0.204%
Atomic mass of Oxygen =.?
The atomic mass of oxygen can be calculated by using the following formula:
Atomic mass = [(Mass of A × A%)/100] + [(Mass of B × B%)/100] + [(Mass of C × C%)/100]
Atomic mass = [(15.995 × 99.759)/100] + [(16.995 × 0.037)/100] + [(17.999 × 0.204)/100]
Atomic mass = 15.956 + 0.006 + 0.037
Atomic mass = 15.999 amu
Therefore, the atomic mass of oxygen is 15.999 amu
Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients.

Answers
The balanced chemical reaction equation is obtained as \(Fe(s) + 2O_{2} (g) + H_{2} O ---- > 2Fe(OH)_{2} (aq)\)
What is a chemical reaction equation?The term chemical reaction equation refers to the way that we represent the combination of the reactants that leads to the formation of the products ins given chemicals reaction process. We know that the stoichiometry of the reaction is very important as it does show us how the reactants and the products can be combined for the particular reaction that we are considering.
We now have the reaction that is occurring between the metallic iron and oxygen and water. This is the reaction that occurs when there is rusting that is occurring on a given or particular piece of iron as we have it here.
We shall now attempt to look at the equation so that the equation that has been shown in the question can be appropriately balanced according to the rules.
Thus, we could write the balanced chemical reaction equation as; \(Fe(s) + 2O_{2} (g) + H_{2} O ---- > 2Fe(OH)_{2} (aq)\)
Learn more about reaction equation:https://brainly.com/question/3588292
#SPJ1
Please can you help me from 1 to 6

Answers
Answer:
um
Explanation:
help you? you mean do it for you? nah man you can just use the calculator ;)
Over the last 20 years, the calorie intake of Americans has increased. This is due in part to
A. increased consumption of fat.
B. the inability to recognize normal serving sizes.
C. the increase in low-price restaurant chains.
D. increased consumption of organic food.
Answers
The amount of calories Americans consume has grown over the past 20 years. This is partly because people are unable to identify typical serving sizes.
According to the set point idea, our DNA is programmed with a predetermined weight baseline for our bodies. This concept indicates that our weight may be limited, as well as the degree by which it deviates from that fixed point. Although some of us have higher weight set points than others, the theory states that our bodies have trouble maintaining these ranges.
Leptin is produced by fat cells and subsequently released into the bloodstream. Leptin reduces a person's desire to eat by acting on specific brain regions. It also appears to have a say in how the body handles its fat reserves.
Learn more about calories:
brainly.com/question/14213632
#SPJ4
What makes the atomic radius change along a period in the periodic table?
A. More electrons in the valence shell make the radius bigger.
B. More protons in the nucleus pull the electrons in, making the
atomic radius smaller.
C. The increased atomic mass makes the atomic radius bigger.
D. More electrons pair in orbitals, making the atomic radius smaller.
Answers
Answer:B
Explanation:
All of the molecules in a cup of water are moving at the same speed or at a variety of speeds explain ?
Answers
Answer:
They will move at the same speed
Explanation:
This will depend on the heat of the water. The hotter the water is the more the molecules move around. As the water cools it will make the molecules move slower.
Hope this helps! :)
what is the polarity of slime molecules
Answers
Slime is a non-Newtonian fluid and does not have a molecular structure that can be considered polar or non-polar. The term "polarity" typically refers to the distribution of electrical charge within a molecule. In the case of slime, the material is made up of polymer molecules, which are long chains of repeating units. These polymer molecules interact with each other through hydrogen bonding, which gives the material its unique properties such as being a non-Newtonian fluid. However, these interactions are not based on polarity, so it is not appropriate to describe slime as being polar or non-polar.
Answer:
The polarity of slime molecules depends on the specific type of slime. There are many different types of slime, each made up of different types of molecules.
For example, if we consider a typical type of slime made from polyvinyl alcohol (PVA), the polarity of the PVA molecules will depend on their molecular structure and the type of bonding between the atoms. PVA is a synthetic polymer made up of repeating units of vinyl alcohol, which has a polar hydroxyl group (-OH). As a result, PVA is considered to be a polar molecule.
However, the overall polarity of a slime made from PVA will also depend on the other ingredients that are present, such as borax, which is often used as a cross-linking agent to form the slime. The overall polarity of the slime will also depend on the conditions it is in, such as the temperature, pH, and the presence of other substances that could affect the polarity of the slime.
In summary, the polarity of slime molecules can be complex and depends on the specific type of slime, the ingredients that make it up, and the conditions it is in.
Explanation:
Just tell me if you kinda confuse
ALLEN
Which one is NOT the name of a measurement system? (1 Point) International System of Units Metric System Spanish System British System
Answers
Spanish System is not the name of a measurement system
WHAT IS MEASUREMENT SYSTEM?
Measurement system are standard systems that are made up of collection is of units of measurement and guiding rules. Measurement system relates numerical values to physical quantities. There are three major system of measurements in use, they are:The international system of units (S.I units)The metric systemThe British imperial systemHence, Spanish System is not one of the names of measurement of systems.
Learn more: https://brainly.com/question/13517246?referrer=searchResults
What is the relationship between an object's mass and its ability to pick up speed, or accelerate?
Answers
Answer:
It is momentum
Since momentum is proportional to the product of mass and velocity
Answer:
The greater the object's mass, the longer it takes to pick up speed or accelerate. There is an inverse relationship between mass and acceleration/picking up speed.
Explanation:
Explain why all other atoms are reactive?
Answers
Answer:
The number of electrons in the outermost shell of an atom determines its reactivity. Noble gases have low reactivity because they have full electron shells. Halogens are highly reactive because they readily gain an electron to fill their outermost shell.
Hope this Helps!~
PLEASE HELP ASAP
Which two reactions are slower than the others?
A. Silver tarnishes in air that contains hydrogen sulfide.
B. Lead nitrate and potassium iodide react to form a yellow
precipitate.
C. Black powder in fireworks burns explosively to produce white
light
D. Iron metal reacts with oxygen in air to form rust.
Answers
Answer:
A and D takes much slower
Explanation:
Here, we want to select, out of the four given reactions, the one that is slower than the other two
The answers in these case are reactions 1 and 4 ( A and D)
The two reactions show what is called rust (as directly seen in reaction 4)
When we speak of rust, we simply mean a reaction that occurs over time
For example, non coated roofings of houses doesn’t get to change color at an instant
The color degradation that occurs is something that takes some time from the initial time they were used to roof the house
Hence, from these analogy, we can see that these reactions need an an external support to thrive or to come into existence
These external supports are natural forces and they contributing efforts occur over time and cannot be seen immediately
These reactions are thus ones that take much slower time than conventional laboratory reactions in the case of the formation of the precipitate or a reaction that requires a low flash point temperature such as that of black powder to produce such explosive effects
So in conclusion, what we are saying is that the two selected reactions are subjected to the availability of some conditions and may take time to manifest and these absolutely differentiates them from reactions that are spontaneous such as the one having an explosive effect or the other one leading to the formation of a precipitate which takes far less times
What are the three states of matter, describe atoms during these states.
Answers
Answer:
Three states of matter is liquid, solid and gas.
I haven't learned atoms, I just learned the states in 5th grade.
Answer:
the three states of matter are
1. solid ( atoms are closely packed )
2. liquid ( atoms are loosely packed )
3. gas ( atoms are very loosely packed )
3. Predict Suppose the chef used two silver
pans instead, but one was three times the
mass of the other. How would the energy
change of the two pans compare?
Answers
The specific heat capacity of a substance is the amount of heat needed to elevate it by 1 degree kelvin per gram. The easier it is for something to heat up, then, the smaller the heat capacity. We would suppose that a cook in a hurry would want a pan that heats up more quickly and would choose one with a lesser heat capacity.
What would be the difference in the two pans' energy changes?0.385 for copper and 0.900 for aluminum.
Despite using non-SI units, we can still compare using it. It is obvious to him that copper has a lower specific heat, so he will pick that.
In case you're curious, you can also approach this issue from the perspective of heat conduction. To do this, look up the thermal conductivities of each material, and then apply Fourier's rule of heat conduction to determine that Copper would be best.
To know more about specific heat visit:-
https://brainly.com/question/11297584
#SPJ1