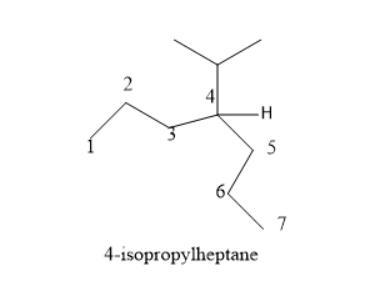
onsider the structure. a carbon is bonded to two three carbon chains and a branched alkane. the branched alkane is a 2 carbon chain with a methyl on carbon 1. select the correct name for the given structure.
Answers
If we consider a structure in which a carbon is bonded to two three carbon chains and a branched alkane then the branched alkane is a 2 carbon chain with a ethyl on Carbon 1 then the correct name of the structure is 4-(isopropyl)heptane.
The two three carbon chains bonded to the carbon atom will form the parent chain of this structure and this chain will have 7 carbon atoms in it.
Since this chain is that of an alkane and it has no functional group attached so the root name will be heptane.
Now, we have to add a prefix by naming the branched alkyl chain. The branched chain has 3 carbon atoms in it with a methyl group on carbon 1.
So, this name of this group will be propyl.
The position of this propyl group is 4th on the parent chain so the full name of the structure will be 4-(isopropyl)heptane.
To know more of branched alkane here
https://brainly.com/question/14507628
#SPJ4
Related Questions
How do the gravitational forces change as the objects get closer to each other?
Answers
Answer:
you can use the example of two magnets
Answer: The gravitational force Increase as the objects get closer.
Explanation:
What is a jet stream. in your own words but be more detailed about what you say.
(don't send a link or file)
Answers
Find the thickness of the material:
This latest alien sample is crazy thin and very tough. After it was cut into something very rectangle-ish with sides of 54.2 cm and 12.3 cm. The calculated density of the material is 1.3 g/cm^3. The mass was found to be 11.4 grams.
Answers
The calculated density of the material is 1.3 g/cm³. The mass was found to be 11.4 grams. The thickness of the material is 14.82 cm³.
What is thickness?Thickness is defined as a dimension, typically the smallest of the three, that describes the separation between two surfaces of an item. There are various layers of fabric that make up a thickness. While density considers how thin or thick strands are collectively, as a group, thickness relates to the breadth of a single hair strand.
Density can be expressed as
Density = mass / volume
Volume = Thickness
So, density = mass / thickness
Thickness = Density x mass
Thickness = 1.3 g/cm³ x 11.4 g
Thickness = 14.82 cm³
Thus, the calculated density of the material is 1.3 g/cm³. The mass was found to be 11.4 grams. The thickness of the material is 14.82 cm³.
To learn more about thickness, refer to the link below:
https://brainly.com/question/11669043
#SPJ1
Tina says, “A solution can have only two different kinds of particles.” Deepa says, “A solution can have many different kinds of particles.” Whom do you agree with? Explain why.
Answers
Answer:
Explanation:
Pick up a soft drink. The CocaCola I have has 8 ingredients and is a mixture. Mind you, it is hard to tell what kind of sugar is present because three are listed.
It certainly has more than 2 ingredients.
What is the mass of a sample of iron that has had 300.0 j applied to it and heats up from 20.0 degrees Celsius to 40.0 degrees Celsius.. the specific heat of iron is 0.46j/gC
Answers
Answer:
The mass of a sample of iron that has had 300 J applied to it and heats up from 20 degrees Celsius to 40 degrees Celsius is 32.61 grams.
Explanation:
Calorimetry is the measurement and calculation of the measurement of heat changes exchanged by a body or a system produced in physical and chemical processes.
The sensible heat of a body is the amount of heat received or transferred by a body to produce a change in temperature but without a change in physical state.
The sensible heat in a constant pressure is calculated by:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, constituted by a substance of specific heat c, and where ΔT is the temperature variation (ΔT=Tfinal - Tinitial)
In this case:
Q= 300 Jc= 0.46 \(\frac{J}{g*C}\)m= ?Tfinal= 40 CTinitial= 20 CReplacing:
300 J= 0.46 \(\frac{J}{g*C}\) * m* (40 - 20) C
Solving:
300 J= 0.46 \(\frac{J}{g*C}\) * m* 20 C
\(m=\frac{300 J}{0.46 \frac{J}{g*C} * 20 C}\)
m= 32.61 g
7. Ammonia can be formed by reacting
nitrogen and nydrogen gases.
N₂(g) + 3H₂(g) → 2NH3
If the rate of disappearance of hydrogen
-2.7 x 10-² what is the rate of formation of ammonia
is
Answers
The rate of formation of ammonia is approximately -1.8 x 10⁻² units (per unit time) based on the given rate of disappearance of hydrogen.
What is the rate of the formation of ammonia?The balanced equation for the reaction is:
N₂(g) + 3H₂(g) → 2NH₃(g)
According to the stoichiometry of the reaction, for every 3 moles of hydrogen (H₂) consumed, 2 moles of ammonia (NH₃) are formed.
Given that the rate of disappearance of hydrogen (-2.7 x 10⁻² is negative, indicating its consumption, we can determine the rate of formation of ammonia using the stoichiometric ratio.
Rate of formation of ammonia = (Rate of disappearance of hydrogen) × (2/3)
Rate of formation of ammonia = (-2.7 x 10^(-2)) × (2/3)
Rate of formation of ammonia ≈ -1.8 x 10^(-2) (units depend on the units of the rate given)
Learn more about rate of reactions at: https://brainly.com/question/12904152
#SPJ1
What’s the ph of the following equation

Answers
Answer: The pH of [H30+] = 2.4 * 10 -3 M is 2.6
Explanation:
pH = 3 - log 2.4 = 2.6
reduction of 1-decyne to decane requires how many equivalents of hydrogen gas?
Answers
The reduction of 1-decyne to decane requires two equivalents of hydrogen gas. This is because the reaction involves the addition of two hydrogen atoms to the triple bond of the 1-decyne molecule, resulting in the formation of a decane.
Each hydrogen atom contributes one electron to form a bond with one of the carbon atoms in the triple bond, and the reaction requires two such additions to convert the triple bond to a single bond. Therefore, two equivalents of hydrogen gas are needed to provide the necessary hydrogen atoms for the reduction to take place.
The reaction is typically carried out using a palladium or platinum catalyst under mild conditions of temperature and pressure. The reduction of alkynes to alkanes is an important organic reaction with many applications in organic synthesis and industrial processes.
You can learn more about reduction at: brainly.com/question/28813812
#SPJ11
A local hamburger shop sold a combined total of 620 hamburgers and cheeseburgers on Wednesday. There were 70 more cheeseburgers sold than hamburgers. How many hamburgers were sold on Wednesday?
Answers
The number of hamburgers sold on Wednesday is 275.
Let's assume the number of hamburgers sold is x.
According to the given information, the number of cheeseburgers sold is 70 more than the number of hamburgers. So, the number of cheeseburgers sold can be expressed as x + 70.
The total number of hamburgers and cheeseburgers sold is given as 620, so we can set up the following equation:
x + (x + 70) = 620
Combining like terms, we get:
2x + 70 = 620
Subtracting 70 from both sides of the equation:
2x = 550
Dividing both sides by 2:
x = 275
Therefore, the number of hamburgers sold on Wednesday is 275.
for more questions on hamburgers
https://brainly.com/question/31214218
#SPJ8
Label the different parts of the atom.

Answers
Answer:(a) electrons
(b) nucleus
(c) protons
(d) neutrons
(e) mass number
Explanation:
What is the oxidizing agent in the reaction Fe+AgNO3-->Fe(NO3)3+AG?
A.AgNO3+
B. fe
C.Ag
D. Fe(NO3)3
Answers
The oxidizing agent in the reaction Fe + \(AgNO_3\)→ \(Fe(NO_3)_3\) + Ag is option a \(AgNO_3.\)
A redox reaction is one in which the oxidation states of two species undergo changes. Iron is oxidized in the reaction, while silver nitrate is reduced. One of the reactants is being reduced, whereas the other is being oxidized.The oxidizing agent is the species that is being reduced, and it is the species that accepts electrons.
Fe is being oxidized in this reaction. Therefore, it cannot be the oxidizing agent, nor can\(Fe(NO_3)_3\). In contrast, \(AgNO_3.\) is being reduced, which means it is accepting electrons. This is why\(AgNO_3.\) is the oxidizing agent.The correct answer is option a.
Know more about oxidizing agent here:
https://brainly.com/question/14041413
#SPJ8
Question.04: (3mrks) A Manometer is a device to measure the pressure of an enclosed d gas sample. A common simple manometer consists of a U shaped tube of glass filled with some liquid. Typically, the liquid is mercury because of its high density. Incandescent light bulbs "burn out" because their tungsten filament evaporates, weakening the thin wire until it breaks. Argon gas is added inside the bulbs to reduce the rate of evaporation. (Argon is chosen because, as a nobi gas, it will not react with the components of the bulb, and because it is easy to obtain in significant quantities. It is the third most abundant element in air.) What is the pressure in atmospheres of 3.4 x 10-³ moles of argon gas in a 75mL incandescent light bulb at 20 °C?
Answers
The pressure of atmospheres of the argon gas in the given incandescent light bulb is 1. 1 .
How to find the pressure of atmospheres ?The pressure of atmospheres can be found by the formula :
= ( Number of moles x Universal gas constant x Temperature in Kelvin ) / Volume of gas
Number of moles = 3.4 x 10 ⁻³
Universal gas constant = 0. 082
Temperature in Kelvin = 20 + 273. 15 = 293. 15 K
Volume of gas : 75 x 10 ⁻³
The pressure of atmospheres of the argon gas is:
= ( 3.4 x 10 ⁻³ x 0. 082 x 293. 15 ) / 75 x 10 ⁻³
= 1. 1 atm
Find out more on pressures of atmospheres at https://brainly.com/question/19587559
#SPJ1
A 50.0 mL sample of 6.0 M HCl was diluted to a final volume of 250.0 mL What was the new molarity?
Answers
Answer:
1.2M
Explanation:
Initial Volume 0.05L
Final Volume 0.250L
HCl Molar mass: 36.46 g/mol
M = 6M HCl
Molarity = mol solute / L of solution
Inital M = Molarity = 6
mol solute = X = unknown
L of Solution = 0.05L
6 = X / 0.05
X = 0.3
X = 0.3/0.25
X = 1.2 M
common things of all metals
Answers
Answer: Well most if not all metals rust due to oxidation. Most can be melted at certain temperatures or just heated to a certain degree to be maluable
Explanation:
causes of majimaji rebellion results
Answers
Answer:
The war was triggered by a German policy designed to force the indigenous population to grow cotton for export and lasted from 1905 to 1907, during which 250,000–300,000 died. After the scramble for Africa among the major European powers in the 1880s, Germany reinforced its hold on several formal African colonies.
Explanation:
please give me brainlist and follow
a flask contains three gases, nitrogen, oxygen, and ammonia. the nitrogen has a partial pressure of 9.65 atm, the oxygen has a partial pressure of 631 torr, and the ammonia has a partial pressure of 1,467 kpa. what it the total pressure in the flask expressed in atm?
Answers
The total pressure in the flask is 24.91 atm.
To calculate the total pressure in the flask, we need to convert the partial pressures of each gas to the same units, preferably atm.
Partial pressure of nitrogen = 9.65 atm
Partial pressure of oxygen = 631 torr = 0.831 atm (since 1 atm = 760 torr)
Partial pressure of ammonia = 1467 kPa = 14.43 atm (since 1 atm = 101.3 kPa)
Now, we can find the total pressure by adding up the partial pressures of each gas:
Total pressure = 9.65 atm + 0.831 atm + 14.43 atm = 24.91 atm
Therefore, the total pressure in the flask is 24.91 atm.
To know more about pressure, refer here:
https://brainly.com/question/30525083#
#SPJ11
Number of valence electrons in sodium
Answers
Answer:
1Explanation:
Sodium Na has 11 electrons.
Sodium has an electronic configuration of 2, 8, 1.
The valence electron is the number of electrons in the last shell.
The number of valence electrons in sodium is 1.
Answer:
Number of valence electrons in sodium is 1 as we know the electronic configuration of sodium that is
\(1s^2,2s^2,2p^6,3s^1\)
as there is only one electron in third sub-shell so the Number of valence electrons in sodium is 1
Explanation:
i hope this will help you :)
Which one of the following elements does not have electrons in an f orbital?
A. Mercury
B. Lead
C. Cesium
Answers
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
Which of the statements best describes the differences between homogeneous and heterogeneous?
Answers
Answer:
See below
Explanation:
A homogeneous solution has the same properties throughout the entire mixture. Water is an example.
Heterogeneous solutions do not. Sand is a good example. Each grain of sand is not identical to the next grain of sand.
What is the molarity of a solution if 41. 50 g potassium iodide (ki) is added to a container that is then filled with water to a final volume of 211 ml?
Answers
Given that 41.50 g of potassium iodide (KI) is added to a container that is then filled with water to a final volume of 211 mL.
We need to find the molarity of the solution. Molarity is defined as the number of moles of solute dissolved in one liter of solution.
In order to find the molarity,
we need to determine the number of moles of KI first.Number of moles of KI = (given mass of KI) / (molar mass of KI) = 41.50 g / 166.0 g/mol = 0.25 mol
Now we can use this to find the molarity .Molarity (M) = (number of moles of solute) / (volume of solution in liters) M = (0.25 mol) / (211 mL / 1000 mL/L) = 1.18 M
Therefore, the molarity of the solution is 1.18 M.
to know more about potassium iodide visit:
brainly.com/question/2809910
#SPJ11
the allowable range for an objective function coefficient assumes that the original estimates for all the other coefficients are completely accurate so that this is the only one whose true value may differ from its original estimate. true or false?
Answers
True. The permissible range for an objective function coefficient represents the degree of uncertainty for the coefficient of interest and is based on the assumption that the initial estimations for all other coefficients in the linear programming model are correct..
The permissible range for an objective function coefficient is predicated on the assumption that all other coefficients in the linear programming model were initially estimated with the highest degree of accuracy. The objective function coefficient, whose true value may vary from its initial estimate, is the sole variable to which the permitted range is thus limited. This is so because linear programming models rely on a number of premises, one of which being that the model's coefficients are known for sure. The acceptable range denotes the range of values that the objective function coefficient can have without undermining the model's underlying assumptions in practise, where these coefficients may be derived using historical data or other sources.
learn more about coefficient here:
https://brainly.com/question/30066987
#SPJ4
PLEASE HURRY!
What is the half-life of a 12 g sample of radioisotope that decayed to 6 g in 28
years?
A. 14 years
B. 7 years
C. 56 years
D. 28 years
Answers
Answer:
28 years
Explanation:
the information is literally in the question.
10.0 mL of 3.0 M sulfuric acid has been added to 50.0 mL of water.
What is the new concentration?
M1V1=M2V2
15 M
1.7 M
0.60M
0.20 M
Answers
Answer:
the new concentration is 0.60M
Explanation:
The computation of the new concentration is shown below;
We know that
M1V1=M2V2
(3.0M) (10.0 mL) = M2 (50.0mL)
30 = M2 (50.0mL)
So, M2 = 0.60 M
Hence, the new concentration is 0.60M
The same is considered and relevant
Why does salt sprinkled on icy roads cause the ice to melt?
Answers
Answer:
The actual reason that the application of salt causes ice to melt is that a solution of water and dissolved salt has a lower freezing point than pure water. When added to ice, salt first dissolves in the film of liquid water that is always present on the surface, thereby lowering its freezing point below the ices temperature. Ice in contact with salty water therefore melts, creating more liquid water, which dissolves more salt, thereby causing more ice to melt, and so on.
where are multivalent ions located on the periodic table
Answers
Multivalent ions are located in the right hand side of periodic table in a zig-zag order.
Multivalent ions are defined as the elements with more than one stable ion. Generally, Ionic compounds containing multivalent elements must have Roman numerals in their names in order to indicate which ions is forming that compound. Basically, the roman numeral is written in brackets after the element to indicate the charge
The Five Multivalent Metals present in the modern periodic table are copper. Cu 1+ copper(I) Cu 2+ copper(II) iron. Fe 2+ iron(II) Fe 3+ iron(III) mercury. Hg2 2+ mercury(I) Hg 2+ mercury(II) lead. Pb 2+ lead(II) Pb 4+ lead(IV) tin. Sn 2+ tin(II) sn 4+ tin(IV).
Learn more about multivalent ions from the link given below.
https://brainly.com/question/30274291
#SPJ4
Write a nuclear equation for the alpha decay of \(^{231} _{91} Pa\)
Answers
Answer:The equation for the alpha decay of is written as .
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle.
The emission of this alpha particle reduces the mass number of the original atom by 4 and the atomic number by 2.
The alpha decay equation is written as;
Explanation: When the given atom () under goes alpha decay, the resulting element is written using the following equation;
Thus, the equation for the alpha decay of is written as .
A gas sample is held at constant pressure. The gas occupies 3.62 L of volume when the temperature is 21.6°C. Determine the temperature at which the volume of the gas is 3.42 L.
A)
312 K
B)
278 K
C)
20.4 K
D)
295 K
E)
552 K
Answers
The temperature at which the volume of the gas is 3.42 L, when held at constant pressure, is 278 K (Option B).
To determine the temperature, we can use Charles's Law, which states that the volume of a gas is directly proportional to its temperature when the pressure is held constant.
The formula for Charles's Law is V1/T1 = V2/T2.
In this case, V1 = 3.62 L, T1 = 21.6°C + 273.15 = 294.75 K, and V2 = 3.42 L.
To find the unknown temperature T2, rearrange the formula as T2 = (V2 * T1) / V1.
Substituting the values, T2 = (3.42 * 294.75) / 3.62 = 278 K. Therefore, the temperature at which the volume of the gas is 3.42 L is 278 K.
Learn more about Charles's Law here:
https://brainly.com/question/21184611
#SPJ11
what happens when carbon dioxide is passed through lime water for a long time? write with equation
Answers
Answer:
when CO2 gas is passed through lime water it turns milky due to the formation of calcium carbonate which formula is CaCO3.
Ca(OH)2+ CO2------ CaCO3
when excess of carbon dioxide is passed through calcium carbonate calcium hydrogen carbonate is formed and solution become colourless.
CaCO3+CO2------ Ca(HCO3)
Early astronomers noticed that some stars in the sky appeared to move together across the sky as constellations. How do astronomers today explain this motion?
The sun's gravity keeps the stars together because they orbit the sun at the same rate.
The sun's gravity keeps the stars together because they orbit the sun at the same rate.
The stars appear to move together along a path because of Earth's rotation on its axis.
The stars appear to move together along a path because of Earth's rotation on its axis.
Gravitational attraction between the stars keeps them fixed relative to each other as they move.
Gravitational attraction between the stars keeps them fixed relative to each other as they move.
The stars move around Earth in the same way, so their positions relative to each other stay the same.
Answers
Answer and Explanation:
Your answer is...
The stars appear to move together along a path because of Earth's rotation on its axis.
Answer:
C. Stars appear to move together along a path because of the earths rotation on its axis.
Explanation:
lol ik its right bc I got a 100 on my science test:)