oxygen (o2) was first isolated by the decomposition of mercury(ii) oxide (hgo) into the mercury and oxygen. if 58.67 g of mercury(ii) oxide were completely decomposed to generate 54.34 g of mercury, how many grams of oxygen should have been produced
Answers
The decomposition of 58.67 grams of mercury(II) oxide (HgO) should have produced 4.33 grams of oxygen (\(O_2\)).
The chemical equation for the decomposition of mercury(II) oxide is:
\(2 HgO \rightarrow 2 Hg + O_2\)
From the equation, we can see that 2 moles of mercury(II) oxide produce 1 mole of oxygen gas. The molar mass of HgO is 216.59 g/mol, while the molar mass of O2 is 32.00 g/mol.
First, we need to calculate the number of moles of HgO that were decomposed:
moles of HgO = mass of HgO / molar mass of HgO
moles of HgO = 58.67 g / 216.59 g/mol
moles of HgO = 0.2709 mol
According to the chemical equation, 2 moles of HgO produce 1 mole of O2. Therefore, the number of moles of O2 produced can be calculated as follows:
moles of \(O_2\) = 0.2709 mol / 2 = 0.1355 mol
Finally, we can calculate the mass of \(O_2\) produced:
mass of \(O_2\) = moles of \(O_2\) x molar mass of \(O_2\)
mass of \(O_2\) = 0.1355 mol x 32.00 g/mol
mass of \(O_2\) = 4.33 g
Therefore, 4.33 grams of oxygen should have been produced.
To learn mole concept refer - https://brainly.com/question/12877841
#SPJ11
Related Questions
SCIENCE
why is it important to distinguish the two types of crust?
Answers
Answer:
It is important to identify it because the two types of crust are made up of two different types of rock
Explanation:
Have a nice day
can someone walk me through how to solve this?

Answers
Which elements occupy the same group in the periodic table?
O A. Elements that share the same row
O B. Elements that share the same inside electrons
O C. Elements that share the same period
O D. Elements that share the same column
Answers
Answer: The answer is D. Elements that share the same column
Explanation:
The elements occupying the same groups are sharing the same column. They have similar chemical and physical properties. Elements of the same group have the same number of valence electrons.
What is group in periodic table?All elements are classified to different groups and periods. The horizontal rows in the table is called periods and the vertical column are called groups in periodic table.
There are 18 groups and 7 periods in periodic table. The atomic number increases from left to right along a period. Elements in a group are having same number of valence electrons and are having similar chemical and physical properties.
Elements in a groups shows similarity in reactivity and physical properties. They show same trend in increasing or decreasing nature of physical quantities. A group is thus, representing a column in periodic table. Therefore, option D is correct.
To find more on groups in periodic table, refer here:
https://brainly.com/question/29761108
#SPJ5
a metal rod weighing 123 g with a temperature of 100 c is placed in a 75 ml of water at 25 c if the final temperature of the metal and water is 29 c what is the specific heat capacity of the metal? assume the density of water is 1.0 g/ml and specific heat capacity of water is 4.184 j/g c
Answers
Answer:
What grade r u in
Explanation:
Just bcauz Ima coo dud and 5=5 and 1=1
what charge does an atom that has gained electrons have?
Answers
Answer:
I think it's positive charge
Sort the following elements in order from least reactive to most reactive.
A - Sb
B - S
C - F
D - As
Answers
Sorting the chemical elements in order from least reactive to most reactive, we have:
1. Fluorine (F).
2. Arsenic (As).
3. Antimony (Sb).
4. Silicon (S).
Given the following chemical elements:
Antimony (Sb).Silicon (S).Fluorine (F).Arsenic (As).Reactivity can be defined as a chemical property which determines how readily a chemical element bonds with other chemical elements, in order to form a new chemical compound.
Generally, the ability of a chemical element to bond with other chemical elements is largely (highly) dependent on the number of valence electrons it has in the outermost shell of its atomic nucleus.
As a general rule, chemical elements that are having fewer number of valence electrons are the most reactive while those having higher valence electrons are least reactive.
Also, chemical reactivity decreases down a group on the periodic table.
Based on the periodic table, the valency for the given chemical elements are:
Antimony (Sb): 5 valence electrons.Silicon (S): 4 valence electrons.Fluorine (F): 7 valence electrons.Arsenic (As): 5 valence electrons.In conclusion, sorting the chemical elements in order from least reactive to most reactive, we have:
1. Fluorine (F).
2. Arsenic (As).
3. Antimony (Sb).
4. Silicon (S).
Find more information: https://brainly.com/question/18214726
From the following list of symbols, choose two elements that would likely form a
molecular compound. Explain your choice.
Mg:
S:
K:
O:
Help plzz
Answers
Answer:
O ( Oxygen )
because oxygen atom is a non metal and highly electronegative which reacts by itself to form a di-molecular compound of Oxygen
\(O _{2}\)
Isotopes of an element cannot have just any number of neutrons. If there are too many or too few, the nucleus will split apart. This is called ____________
Answers
Answer:
beta decay
Explanation:
Which statement describes why sodium reacts more vigorously than magnesium to hydrochloric acid? Refer to the periodic table. Circle the letter of the correct answer.
1) Sodium has a smaller atomic radius than magnesium.
2)Sodium has a lower ionization energy than magnesium.
3)Magnesium has a lower ionization energy than sodium.
Answers
Answer:
Its B all the others arent true and also dont make any sense
Explanation:
Sodium has a lower ionization energy than magnesium describes why sodium reacts vigorously than magnesium chloride.
Why is sodium more reactive than magnesium?Sodium is more reactive than magnesium because it has the ability to easily lose electron, hence have lower ionization energy.Sodium belong to group one on the periodic table and they are called akali metal while magnesium belong to group two on the periodic table and they are called alkali Earth metal.Sodium and magnesium belong to the in the 3rd period. Iin the outermost energy level sodium has one electron but magnesium has 2 electrons. Therefore, there is more attraction abetween the nucleus and electrons in magnesium than that of sodium.Therefore, sodium is more reactive than magnesium chloride because of lower ionization energy.
For more details on sodium reactivity, check the link below.
https://brainly.com/question/6837593
Imagine that you are an astronomer and you have detected a star that has a temperature of about 3700 Kelvin, and a luminosity of about 0.1. examine the H-R diagram explain what spectral class and part of its life cycle the star could be in ?
Answers
Answer:
Annie Jump Cannon entered Wellesley College in Massachusetts in 1880 to study astronomy. She became interested in stellar spectroscopy, the process of breaking light from stars down into its component colors so the various elements can be identified. After suffering from scarlet fever, which left her hearing impaired, she earned her master�s degree and then continued her studies at Radcliffe College. She became an assistant at the Harvard College Observatory, the first observatory to include women as staff members. During her career, she observed, classified, and analyzed the spectra of some five hundred thousand stars, assigning each one its place in the sequence O, B, A, F, G, K, and M. In 1911 she almost became a faculty member at Harvard but the university officials refused to promote a woman to such high status. So she became the curator of astronomical photographs, earning a salary of twelve hundred dollars a year. Finally, in 1936, Harvard hired her as a permanent faculty member. She was seventy-three years old at the time.
Annie Jump Cannon entered Wellesley College in Massachusetts in 1880 to study astronomy. She became interested in stellar spectroscopy, the process of breaking light from stars down into its component colors so the various elements can be identified. After suffering from scarlet fever, which left her hearing impaired, she earned her master�s degree and then continued her studies at Radcliffe College. She became an assistant at the Harvard College Observatory, the first observatory to include women as staff members. During her career, she observed, classified, and analyzed the spectra of some five hundred thousand stars, assigning each one its place in the sequence O, B, A, F, G, K, and M. In 1911 she almost became a faculty member at Harvard but the university officials refused to promote a woman to such high status. So she became the curator of astronomical photographs, earning a salary of twelve hundred dollars a year. Finally, in 1936, Harvard hired her as a permanent faculty member. She was seventy-three years old at the time.Astronomers now realize that everything which appears to distinguish one star from another - temperature, luminosity, size, life span -- is determined almost entirely by one factor: the star's mass. The main sequence along the HR diagram is not a singular evolutionary path, as many had thought, but a portrait of the sky at one moment in time of stars with varying masses.
Annie Jump Cannon entered Wellesley College in Massachusetts in 1880 to study astronomy. She became interested in stellar spectroscopy, the process of breaking light from stars down into its component colors so the various elements can be identified. After suffering from scarlet fever, which left her hearing impaired, she earned her master�s degree and then continued her studies at Radcliffe College. She became an assistant at the Harvard College Observatory, the first observatory to include women as staff members. During her career, she observed, classified, and analyzed the spectra of some five hundred thousand stars, assigning each one its place in the sequence O, B, A, F, G, K, and M. In 1911 she almost became a faculty member at Harvard but the university officials refused to promote a woman to such high status. So she became the curator of astronomical photographs, earning a salary of twelve hundred dollars a year. Finally, in 1936, Harvard hired her as a permanent faculty member. She was seventy-three years old at the time.Astronomers now realize that everything which appears to distinguish one star from another - temperature, luminosity, size, life span -- is determined almost entirely by one factor: the star's mass. The main sequence along the HR diagram is not a singular evolutionary path, as many had thought, but a portrait of the sky at one moment in time of stars with varying masses.Below is a version of the Hertzsprung-Russell diagram, which shows how the
Explanation:
MARK ME AS A BRAINLIST PLZ
The photoelectron spectra for H and He are represented at left. Which of the following statements best accounts for the fact that the peak on the He spectrum is farther to the left and higher than the peak on the H spectrum?answer choicesHe has an additional valence electron in a higher energy level than the valence electron in H .He has a greater nuclear charge than H and an additional electron in the same energy level.He has a completely filled valence shell in which the electrons are a greater distance from the nucleus than the distance between the H nucleus and its electron.It takes longer for the electrons in He to be removed due to the higher nuclear mass of He.
Answers
The answer is Option b .He has a greater nuclear charge than H and an additional electron in the same energy level
What is Photoelectron Spectroscopy (PES)?PES (photoelectron spectroscopy) is a method for figuring out how much energy electrons in atoms and molecules have relative to one another. PES is frequently used by scientists to examine molecular bonding or to find out what elements make up a material.
By ionising a sample and examining the kinetic energy distribution of the released photoelectrons, a technique called photoelectron spectroscopy (PES) investigates the composition and electronic state of the sample's surface region.By measuring the kinetic energy of photoelectrons to ascertain their binding energy, intensity, and angular distributions, it is possible to analyse the electronic structure of molecules. By detecting electrons rather than photons, it studies a substance's electrical structure, which sets it apart from traditional spectroscopy.The relative energy of electrons in atoms and molecules can be ascertained via photoelectron spectroscopy (PES). A PES spectrum is a graph of the photoelectron count vs binding energy.The electrons in an atom's various subshells are represented by PES peaks. The peaks with the lowest binding energies correspond to valence electrons, while the peaks with the highest binding energies correspond to core electrons.The photoelectron spectra of typical hydrogen-bonded complexesThe photoelectron spectra of typical hydrogen-bonded complexes had been seen in the gas phase. It was discovered that whereas the three higher occupied orbitals of the proton donor are destabilized by hydrogen-bond formation, the nonbonding orbital of the proton acceptor is greatly stabilized. The charge-transfer concept for the hydrogen bond is strongly supported by the significant orbital energy shifts brought on by hydrogen bond formation.
Eg : CF3COOH–(C2H5)2NCH3, CF3COOH–(n-C3H7)3N, CF3CF2COOH–(C2H5)2NCH3, and CF3CF2COOH–(n-C3H7)3N
To know more about photoelectron spectra refer to :
https://brainly.com/question/25267913
#SPJ4
Would you expect to find clear weather or clouds In Fargo, North Dakota
Answers
Answer:
Basically, weather
Explanation:
Because if you go to north Dakota, you will see some weather up there
An old refrigerator is rated at 500 W how many kilowatt hours of electric energy what does refrigerator use in 30 days assume the refrigerator is running 12 hours per day
Answers
The refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
To calculate the kilowatt-hours (kWh) of electric energy used by the refrigerator in 30 days, we need to multiply the power rating by the total running time.
Given:
Power rating of the refrigerator = 500 W
Running time per day = 12 hours
Number of days = 30
First, we need to convert the power rating from watts to kilowatts:
Power rating = 500 W / 1000 = 0.5 kW
Next, we calculate the total energy used in kilowatt-hours (kWh) over the 30-day period:
Energy used = Power rating × Running time × Number of days
Energy used = 0.5 kW × 12 hours/day × 30 days
Energy used = 180 kWh
Therefore, the refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
For more question on energy
https://brainly.com/question/29339318
#SPJ8
the ion responsible for propagation of the ap across the synapse is
Answers
The ion responsible for the propagation of the action potential (AP) across the synapse is the calcium ion (Ca2+). In a neuron, the AP is an electrical signal that travels along the axon towards the synapse, which is the junction between two neurons.
The propagation of the AP involves the opening and closing of voltage-gated ion channels, allowing for the flow of sodium (Na+) and potassium (K+) ions across the neuron's membrane, resulting in a change in the membrane potential.
When the AP reaches the synaptic terminal, it causes the opening of voltage-gated calcium channels, allowing calcium ions (Ca2+) to enter the neuron. The influx of calcium ions into the synaptic terminal triggers the release of neurotransmitters, which are stored in vesicles.
The neurotransmitters are then released into the synaptic cleft, the small gap between the pre-and post-synaptic neurons. The released neurotransmitters bind to specific receptors on the post-synaptic neuron's membrane, causing ion channels to open and allowing the flow of specific ions, such as sodium or potassium, into or out of the post-synaptic neuron.
This creates a new electrical signal, known as the post-synaptic potential, which can either excite or inhibit the post-synaptic neuron. If the post-synaptic potential reaches a certain threshold, it can generate a new action potential in the post-synaptic neuron, allowing for the propagation of the signal across the synapse and the continuation of neural communication.
To know more about action potential refer here:
https://brainly.com/question/12965263#
#SPJ11
Help ASAP.................

Answers
Answer:
Abiotic and Biotic factors?
how many atoms are in hydrogen
Answers
Answer:6.02
Explanation:
Answer:
there are about 6 atoms in hydrogen
diamonds and graphite are two forms of what non metal
Answers
Diamonds and graphite are two forms of the nonmetal carbon.
Diamonds:
Diamond is a crystalline form of carbon, known for its exceptional hardness and brilliant luster. It is composed of carbon atoms arranged in a rigid three-dimensional lattice structure, with each carbon atom bonded to four neighboring carbon atoms through strong covalent bonds. This arrangement gives diamonds their characteristic strength and makes them the hardest known naturally occurring substance. Diamonds are transparent and prized for their use in jewelry, industrial applications, and as a symbol of luxury.
Graphite:
Graphite is another form of carbon with a distinct structure and properties. Unlike diamonds, graphite is composed of carbon atoms arranged in layers or sheets, where each carbon atom is bonded to three neighboring carbon atoms. Within the layers, the carbon atoms form strong covalent bonds, but there are weak forces between the layers called van der Waals forces. These forces allow the layers to slide over one another, making graphite soft and slippery. Graphite is opaque, grayish-black in color, and has a greasy or slippery feel. It is commonly used as a lubricant, in pencils, as a heat-resistant material, and in various industrial applications.
Both diamonds and graphite are forms of carbon, but their distinct structures result in vastly different properties and applications. Diamonds are known for their hardness and brilliance, while graphite is known for its softness and lubricating properties.
to know more about carbon visit:
brainly.com/question/32339967
#SPJ11
HELP PLEASE!! BRAINLIEST!! 5 STARS & THANKS

Answers
step by step explanation
What is the concentration of lithium ions in 0.350 M Li₃PO₄?
Answer ASAP DUE TODAY
Answers
we will measure the amount of lithium ion, Li+, present in 0.4 M Li2HPO4. This is attainable as follows: 1 mole of Li2HPO4 resulted in 2 moles of product in the equation for balance above.
What is the purpose of lithium?
Although its activities are unknown, trace levels of lithium are found in biological systems. Lithium salts have shown potential for the treatment for mental illnesses including bipolar disorder as an antidepressant and mood stabilizer. The lithium family, named for its main element, is another name for the alkali metals.
A lithium battery is what?
The most effective of them allows migration of a lithium cation, Li +, between the anode and the cathode, such as LiCoO 2, using a conducting polymer without a solvent. Cell phones, laptops, and other electronics frequently use smaller rechargeable lithium batteries.
To know more about lithium visit:
https://brainly.com/question/1439744
#SPJ1
Carbon burns with oxygen to produce carbon dioxide gas.
Which of these shows the correct chemical reaction?
O C+0=CO2
O CO, CO2
C +02 + CO2
CO2 = C+0
Answers
Answer:
it will help you , but I am not sure it is correct

Given the blackbody curve of the graph, what color will Star A be?
Blackbody radiation graph for Star A, which has a surface temperature of 17,000 degrees Celsius. At the point where the curve peaks, the wavelength is approximately 400 nanometers, at the far-left end of the visible light spectrum.
Blue
Red
White
Yellow
Answers
Star A will have blue color as hottest stars are blue in color.
Effect of temperature Because it reveals the star's surface temperature on the scale of black body radiation, a star's hue is essential for identifying it. 5,500 K is usual for a yellow star and is the temperature of the sun's surface. A bright red star's surface temperature is 3,500 K, while a dark red star's surface temperature is 2,500 K. Red stars are cooler than the sun. With surface temperatures ranging from 10,000 K to 50,000 K, blue stars are the hottest stars known to exist.Nuclear fusion events at stars' cores provide the energy for them to shine. Throughout the course of the star's life, a dynamic equilibrium is maintained between the gravitational forces that keep the star together and the reactive core's expanding heat. High levels of energy are produced through fusion.For more information on color of star kindly visit to
https://brainly.com/question/7513288
#SPJ1
what type of reaction is CO2 + H2O–H2CO3?
Answers
What is the correct order of the scientists in order of their work related to the structure of an atom from earliest to most recent?
Neils Bohr, Ernest Rutherford, JJ Thomson, John Dalton, Democritus and Erwin Schrodinger
Erwin Schrodinger, JJ Thomson, John Dalton, Neils Bohr, Ernest Rutherford and Democritus
Democritus, John Dalton, JJ Thomson, Ernest Rutherford, Neils Bohr and Erwin Schrodinger
John Dalton, Neils Bohr, JJ Thomson, Democritus, and Ernest Rutherford and Erwin Schrodinger
PLEASE HELP !!!
Answers
Answer:
C. democritusm John dalton, JJ Thomason, Ernest Rutherford, Neils Bohr, and Erwin Schrodinger.
The correct order of the scientists in order of their work related to the
structure of an atom from earliest to most recent include Democritus, John
Dalton, JJ Thomson, Ernest Rutherford, Neils Bohr, and Erwin Schrodinger.
Democritus was regarded as one of the earliest scientist who first proposed
atomic theory around 460 BC which was then modified and used by others
such as John Dalton, JJ Thomson, Ernest Rutherford and Neils Bohr.
The most recent of them all was Erwin Schrodinger which was the wave
equation for electron movements in the 20th century.
Read more on https://brainly.com/question/20339664
What’s the answer??????
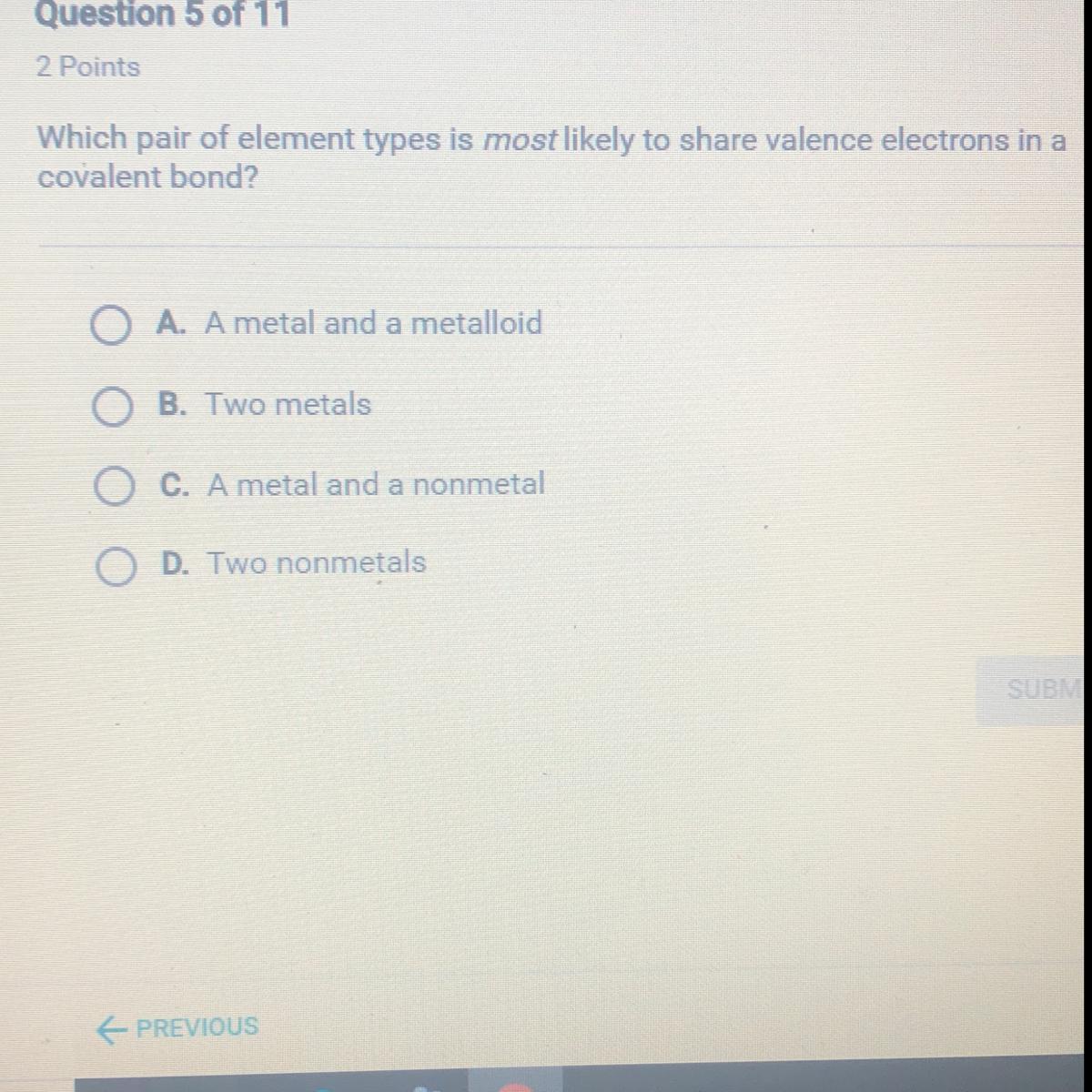
Answers
Answer:
D. Two non-metals
Explanation:
Non metals mostly form covalent bonds where they share electrons whereas, metal and nonmetal form ionic bonds where electrons are transferred.
Which equation is derived from the combined gas law?
StartFraction V subscript 1 over T subscript 1 EndFraction equals StartFraction V subscript 2 over T subscript 2 EndFraction.
StartFraction V subscript 1 over T subscript 2 EndFraction equals StartFraction V subscript 2 over T subscript 1 EndFraction.
V subscript 1 T subscript 1 equals P subscript 2 T subscript 2.
P subscript 1 V subscript 1 T subscript 1 equals P subscript 2 V subscript 2 T subscript 2.
Answers
The equation derived from the combined gas law is option D: P₁V₁T₁ = P₂V₂T₂. Option D
The combined gas law combines Boyle's law, Charles's law, and Gay-Lussac's law into a single equation that relates the pressure, volume, and temperature of a gas sample. It allows us to analyze changes in these variables while keeping the amount of gas constant.
Boyle's law states that at a constant temperature, the pressure and volume of a gas are inversely proportional. In other words, if the volume of a gas decreases, its pressure increases, and vice versa. This is expressed as P₁V₁ = P₂V₂.
Charles's law states that at a constant pressure, the volume and temperature of a gas are directly proportional. If the temperature of a gas increases, its volume increases, and vice versa. This is expressed as V₁/T₁ = V₂/T₂.
Gay-Lussac's law states that at a constant volume, the pressure and temperature of a gas are directly proportional. If the temperature of a gas increases, its pressure increases, and vice versa. This is expressed as P₁/T₁ = P₂/T₂.
By combining these three laws, we obtain the combined gas law equation: (P₁V₁)/T₁ = (P₂V₂)/T₂. To eliminate the division, we can cross-multiply to get P₁V₁T₂ = P₂V₂T₁, which can be rearranged as P₁V₁T₁ = P₂V₂T₂.
This equation allows us to calculate the final values of pressure, volume, or temperature when any two of these variables change while the amount of gas remains constant. It is particularly useful in analyzing the behavior of gases under different conditions or when studying gas systems.
Option D
For more such question on gas law visit:
https://brainly.com/question/27870704
#SPJ8
Answer:
it was A for me.. don't know if this will help
Explanation:
The chart shows the time, initial velocity, and final velocity of three riders.
A 4-column table with 3 rows. The first row labeled rider has entries Gabriella, Franklin, Kendall. The second row labeled time with entries 10 seconds, 8.5 seconds, 6 seconds. The third column labeled initial velocity has entries 55, 50, 53.2. The fourth column labeled final velocity has entries 32, 50, 67.
Which best describes the riders' final velocities ?
Gabriella is speeding up at the same rate that Kendall is slowing down, and Franklin is not accelerating.
Gabriella is slowing down at the same rate that Kendall is speeding up, and Franklin is not accelerating.
Gabriella and Franklin are both slowing down, and Kendall is accelerating.
Gabriella is slowing down, and Kendall and Franklin are accelerating.
Answers
Answer:
Gabriella is slowing down at the same rate that Kendall is speeding up, and Franklin is not accelerating.
Gabriella is slowing down at the same rate that Kendall is speeding up, and Franklin is not accelerating. Hence option B is correct.
What is acceleration?Acceleration is defined as the speed at which an object's velocity changes over time. The rate at which an item changes its velocity is known as acceleration, a vector quantity. If an object's velocity is changing, it is accelerating. Acceleration can be expressed as
Acceleration = Displacement / Time
Velocity is defined as the pace at which an item changes position while moving in one direction, as seen from a certain point of view and as measured by a specific unit of time. Velocity is the pace and direction of an object's movement, whereas speed is the time rate at which an object is travelling along a path.
Thus, Gabriella is slowing down at the same rate that Kendall is speeding up, and Franklin is not accelerating. Hence option B is correct.
To learn more about acceleration, refer to the link below:
https://brainly.com/question/12550364
#SPJ6
NaCl solution is an example of a/an ____________.A. ConductorB. MetalloidC. InsulatorD. Nonmetal
Answers
When NaCl is placed in water, it becomes an aqueous solution, one thing that occurs in this situation is that NaCl, which is an ionic compound, will dissociate into Na+ and Cl-, this dissociation into ions will cause the solution to become a good conductor of electricity. Therefore the correct answer will be letter A
Temperature __________ as the average kinetic energy of a gas increases. stays the same decreases increases
Answers
Answer:
increases
Explanation:
Temperature is essentially the measure of average kinetic energy, so the higher the average kinetic energy, the higher the temperature.
which observation best describes the physical appearance of a compound when the end of its melting point range is reached? the compound begins to convert to a liquid. the compound completely converts to a liquid. the compound begins to evaporate.
Answers
A compound turns completely into a liquid this observation best describes the physical appearance of a compound when it reaches the end of its melting point range. Here option B is the correct answer.
When a solid compound is heated, it undergoes a process called melting in which it transforms into a liquid state. The melting point of a compound is the temperature at which it changes from a solid to a liquid state. The melting process is characterized by a range of temperatures over which the compound is observed to be partially or fully melted.
The observation that best describes the physical appearance of a compound when the end of its melting point range is reached is B - the compound completely converts to a liquid. At the end of the melting point range, the compound has absorbed enough heat energy to fully overcome the intermolecular forces that hold its constituent particles together in a solid state, resulting in the complete transformation of the compound into a liquid.
This state is characterized by the loss of a crystalline structure, where the particles are free to move about and slide past each other, leading to an increased fluidity and mobility of the compound. At this stage, the compound is fully melted and can be poured or transferred into a new container in its liquid form.
To learn more about melting points
https://brainly.com/question/28902417
#SPJ4
Complete question:
Which observation best describes the physical appearance of a compound when the end of its melting point range is reached?
A - the compound begins to convert to a liquid.
B - the compound completely converts to a liquid.
C - the compound begins to evaporate.
What is the [H+] in a solution with pOH of 0.253? A. 5.58 × 10−15 M B. 1.79 × 10−14 M C. 3.21 × 10−2 M D. 5.58 × 10−1 M
Answers
Answer:
The answer is option BExplanation:
To find [H+] in the solution we must first find the pH
That's
pH = - log[H+]
pH + pOH = 14
pOH = 0.253
pH = 14 - 0.253
pH = 13.747
Since we've found the pH we can now find the [H+] in the solution
We have
pH = - log[H+]
13.747 = - log[H+]
Take antilog of both sides
We have the final answer as
\([H+] = 1.79 \times {10}^{ - 14} M\)
Hope this helps you
Answer:
Okay so we know that pOH + pH = 14, so if pOH is 0.253 the pH would be 13.747.
And pH = -log [H+], so [H+] = 10^(-pH) -->This is just the antillog...
so [H+] = 1.791e-14, which would make sense for the strongly basic solution (so B is the correct answer)
Hope this helps...