Step 2: Determine which of the carbocations formed is the major intermediate, First characterize each carbocation. H H carbocation A carbocation B Answer Bank secondary primary tertiary allylic dis the tion H u H ation B carbocation C carbocation D Answer Bank lylic tertiary allylic tertiary primary Draw the kinetic and thermodynamic addition products formed when one equivalent of HBr reacts with the diene shown. X carbocation A carbocation B Strategy Step 1: Draw the carbocations formed from addition of proton to each alene. Step 2: Classify the carbocations and determine the major intermediate Step 3: Draw the resonance structure for the major intermediate Step 4: Draw the 1.2 and 1,4 addition products. Step 5: Identify the kinetic and thermodynamic products, Answer Ba secondary secondary allylic The most stable carbocation is
Answers
The most stable carbocation is the tertiary carbocation, carbocation B.
Tertiary carbocations are the most stable type of carbocation due to having the most delocalization of charge, which reduces the energy of the system and makes it more stable.
This occurs due to having three alkyl groups on the carbon atom bearing the charge, allowing for the positive charge to be delocalized over three atoms,
thereby reducing the repulsive forces between the positively charged atoms.
Additionally, having three alkyl groups helps to increase the electron density around the carbon bearing the positive charge, further stabilizing the system.
The kinetic product of the reaction between one equivalent of HBr and the diene shown is an allylic carbocation, which is the intermediate formed during the reaction.
This is due to the reaction between the proton of the HBr and the double bond of the diene forming an allylic carbocation.
This allylic carbocation is relatively unstable compared to the tertiary carbocation, carbocation B, and thus is not the major intermediate.
The thermodynamic product of the reaction is a 1,4 addition product, which is the product that is most stable and therefore the thermodynamic product.
This 1,4 addition product is formed from the addition of the proton of the HBr and the lone pair of electrons of the double bond to the opposite sides of the double bond.
The most stable carbocation in this reaction is the tertiary carbocation, carbocation B, which is formed from the protonation of the double bond.
This is due to the delocalization of charge over three atoms and the increased electron density around the positively charged carbon.
The kinetic product is an allylic carbocation, while the thermodynamic product is a 1,4 addition product.
to know more about carbocation refer here:
https://brainly.com/question/13164680#
#SPJ11
Related Questions
How does an indicator differentiate between an acid and base?
Changes solubility
Changes reactivity
Changes color
Changes size
Answers
Answer:
that is all I know sorry hope that may help you :)
Explanation:
Indicator is a substance which shows different colors in acidic and basic medium. Indicators can be natural (derived from natural sources) or artificial ( man-made) for example :
*Litmus is an indicator. Acid turns blue litmus into red while base turns red litmus into blue
* Turmeric solution does not show change in color (remains yellow) in acidic solution and turns red in basic solution.
NEED HELP ASAP!!!!
Which process do scientists think provided Earth with an oxygen-rich atmosphere?
radiometric dating
spontaneous generation
photosynthesis
respiration
Answers
Correct me if I’m wrong
Answer:
photosynthesis
Explanation:
took the test
fluorine, chlorine, bromine, and iodine all have the same number of valence electrons and have a tendency to gain electrons. which element has the greatest ionization energy and electronegativity?iodinebchlorinecfluorinedbromine
Answers
Fluorine.Accordingly the order of electronegativity of the given elements would be: Fluorine > Chlorine > Bromine > Iodine. ( Fluorine has the highest electronegativity.)
Fluorine and Chlorine (Atomic numbers 9 and 17) are situated in similar segment of the periodic table of elements. This truly intends that: fluorine and chlorine have similar number of electrons it their external shells. fluorine and chlorine have similar number of all out electrons.The elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) are incandescent light. Incandescent light are profoundly reactive nonmetallic elements in bunch 17 of the periodic table. They have seven valence electrons, so they are very "energetic" to acquire one electron to have a full external energy level.
Find more about Electronegativity
brainly.com/question/862584
#SPJ4
Is this statement true or false?
Decomposers such as bacteria recycle dead tissue into reusable chemicals for other producers. Then the consumers eat the producers. This is an example of how nutrients are recycled through an ecosystem
Answers
It is true that decomposers such as bacteria recycle dead tissue into reusable chemicals for other producers, which are then eaten by consumers.
What is nutrient recycling?Nutrient recycling is the process by which essential elements in the ecosystem are in transition between abiotic and biotic factors.
In an ecosystem, the following groups of organisms exists:
Producers e.g plantsConsumers e.g. herbivores, carnivoresDecomposers e.g. bacteria, fungiDecomposers are responsible for breaking down dead tissues of living organisms leading to the release of nutrients to the soil. These nutrients are used by producers, which are then eaten by consumers.
Learn more about nutrient recycling at: https://brainly.com/question/1410462
The number of neutrons present in the nucleus of an average atom of any given element is best estimated by:_______-
Answers
The number of neutrons present in the nucleus of an average atom in any given element is best estimated by subtracting the number of protons from the mass number.
All atoms contain protons, neutrons and electrons, although the amount differs for each atom. Neutrons are located in the nucleus of each atom. They were first discovered by James Chadwick. They are also neutral and have no charge.
Apart from neutrons, positively charged protons are present in the nucleus. The number of protons is equal to the atomic number, usually denoted with the letter Z.
The mass number or atomic mass is the total mass of the atom. It is calculated by adding the number of protons to the number of neutrons. Electrons are really light and have negligible mass (about 1/1840 times the mass of a proton), so we don't add their masses when calculating mass number. So, to get the number of neutrons in an atom, subtract the number of protons from the mass number.
Learn more about atoms here:
https://brainly.com/question/1379579
#SPJ4
How many hydrogen atoms are in 4 molecules of H2O?
Answers
Answer: 8
Explanation:
Answer:
I think the answer is 4 or 3, it could be 2 as well, I'm not sure.
Explanation:
octane ratings are being discussed. technician a says most modern engines are designed to use regular grade gasoline. technician b says to use high octane gasoline only when an engine was designed to use it. who is correct?
Answers
Both Technician A and Technician B are correct in their statements about octane ratings and the use of regular and high octane gasoline.
Technician A is correct because most modern engines are designed to use regular grade gasoline, which has an octane rating of 87. This is the most commonly used gasoline and it is suitable for most engines.
Technician B is also correct because high octane gasoline, which has an octane rating of 91 or higher, should only be used when an engine was designed to use it. High octane gasoline is more expensive and is not necessary for most engines. However, some high-performance engines require high octane gasoline to prevent engine knocking and to achieve optimal performance.
Therefore, both Technician A and Technician B are correct in their statements about octane ratings and the use of regular and high octane gasoline.
To know more about Octane Gasoline:
https://brainly.com/question/29488465
#SPJ11
how do you find the metling point of a tin with the mass of 232
Answers
The melting point of tin is approximately 231.93 degrees Celsius or 449.47 degrees Fahrenheit. This means that when tin reaches this temperature, it becomes a liquid.
Melting of a substance happens when temperature it's at makes it change it's state from solid to liquid. For tin, this temperature would be 231.93 degrees Celsius or 449.47 degrees Fahrenheit. When tin is heated to this temperature, the forces holding its inner particles weaken, allowing it to transform from a solid tin to a liquid state
It is important to note that the temperature may vary slightly depending on the purity of the tin and other factors.
To know more about Melting Points : https://brainly.com/question/40140
please im even confused with what to do ;-;

Answers
Answer:
Explanation:
The C option is the correct answer
Answer:
1) MEASURING CYLINDER as the name suggest it is cyllindrical also it is used in measure the volume of a liquid. It has a narrow cylindrical shape
2) BURRETE a graduated glass tube with a tap at one end, for delivering known volumes of a liquid, especially in titrations.
3) Pipette To transport a measured volume of liquid
SO THE ANSWER IS C, hope it helps
How many moles are present in 10.0 grams of
sodium hydroxide
Answers
The number of moles present in 10.0grams of sodium hydroxide is 0.25moles.
How to calculate number of moles?The number of moles in a substance can be calculated by dividing the mass of the substance by its molar mass as follows:
no of moles = mass ÷ molar mass
The number of moles in chemistry is the base unit of amount of substance i.e. the amount of substance of a system which contains exactly 6.02214076 × 10²³ elementary entities.
molar mass of sodium hydroxide = 40g/mol
moles = 10g ÷ 40g/mol
moles = 0.25moles
Therefore, 0.25 moles is the amount of moles in the compound.
Learn more about moles at: https://brainly.com/question/14919968
#SPJ1
Answer:
Explanation:m
Which gas will have the greatest volume at STP?
A) 22.4 L of nitrogen gas
B) 5 g of hydrogen gas
C) 1 mole of helium
D) 10 g of oxygen gas
Answers
Answer:
1 mole of Helium is answer
how many protons are in an atom of strontium-90
Answers
An atom of strontium-90 has 38 protons because the number of protons in an element is determined by its atomic number, which is always a whole number. The atomic number of strontium is 38, which means that a neutral strontium atom has 38 protons in its nucleus.
The "90" in strontium-90 refers to its atomic mass, which is the sum of its protons and neutrons. Since strontium-90 has a mass number of 90, and strontium has an atomic number of 38, we can calculate the number of neutrons by subtracting 38 from 90, which gives us 52 neutrons. Therefore, the nucleus of strontium-90 contains 38 protons and 52 neutrons.
To know more about strontium-90 click this link -
brainly.com/question/21177675
#SPJ11
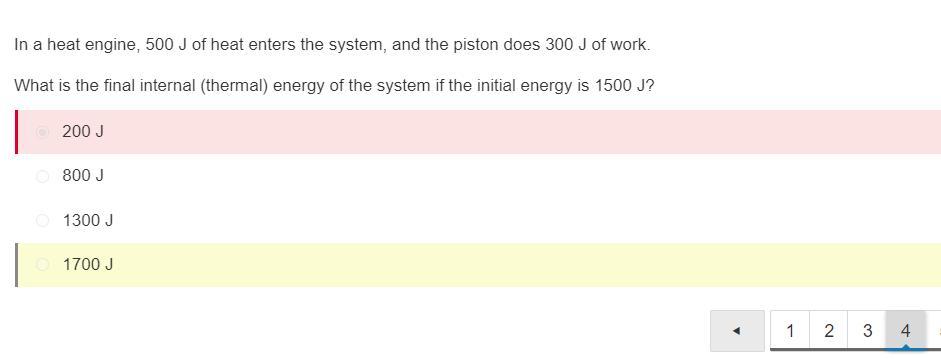
In a heat engine, 500 J of heat enters the system, and the piston does 300 J of work. What is the final internal (thermal) energy of the system if the initial energy is 1500 J?
200 J
800 J
1300 J
1700 J
Answers
Answer:
200J
Explanation:
This is because 500J - 300J = 200J
I hope this helps!!!
Answer:
1700 Joules. I Took the test.
Explanation:
See image for question

Answers
Answer:
the energy produced as part of the reaction and balancing the equation accounts for the energy produced
Which statement describes what happens when somebody slaps their hand on a wall?

Answers
Answer:
B. Wall applies equal reaction force on hand
Explanation:
This is newton's third law. You push something, that something pushes you back with same force. The direction is opposite, so if I push a wall, the wall pushes me back with same force just in opposite direction (so wall towards me). This is why option A is incorrect b/c it says force in same direction as wall. C and D talk about unequal force which is incorrect.
Answer:Wall applies equal reaction force on hand
Explanation:
learn more about the role of secretin in regulating pancreatic bicarbonate ( hco3− ) secretion by completing each sentence using the terms provided.
Answers
Secretin is a hormone released by the duodenal mucosa in response to acidic chyme. It binds to pancreatic ductal cell receptors, initiating a signaling cascade that activates adenylate cyclase.
This leads to increased cyclic AMP (cAMP) levels, promoting the secretion of bicarbonate (HCO3-) ions by pancreatic ductal cells. Bicarbonate ions are transported across the apical membrane into the pancreatic ducts, where they combine with protons to form carbonic acid. Carbonic acid is rapidly converted to water and carbon dioxide by carbonic anhydrase. Carbon dioxide can diffuse into the bloodstream, while remaining bicarbonate ions exit the basolateral membrane into the bloodstream. This secretion of bicarbonate helps neutralize acidic chyme in the duodenum, maintaining a suitable pH for digestive enzyme activity and protecting the intestinal mucosa.
To know more about secretion , visit:
https://brainly.com/question/33292633
#SPJ11
Three safety-related rules concerning the location of machine controls on equipment involving fluid power components.
Answers
1. Ensure Clear and Visible Placement: Machine controls should be located in a position that is easily accessible, visible, and within reach of the equipment operator. Clear and intuitive labeling or color-coding can also be used to enhance visibility and assist in identifying the controls quickly.
2. Provide Adequate Guarding: The machine controls should be positioned in a manner that minimizes the risk of accidental activation or unintended operation. This can be achieved by incorporating appropriate guarding or barriers around the controls to prevent inadvertent contact or interference.
3. Consider Ergonomics and Operator Comfort: When determining the location of machine controls, it is essential to consider ergonomic principles and operator comfort. Controls should be positioned in a way that allows operators to maintain a comfortable and natural posture while operating the equipment. This can help reduce the risk of operator fatigue, musculoskeletal disorders, and errors due to discomfort or awkward reach.
These rules aim to promote operator safety, minimize the potential for accidents, and ensure efficient and effective control of equipment involving fluid power components.
To know more about musculoskeletal disorders.
https://brainly.com/question/30279097
#SPJ11
which of the following metals will not dissolve in nitric acid or hydrochloric acid? which of the following metals will not dissolve in nitric acid or hydrochloric acid? fe mg k ca all of the above will dissolve
Answers
Potassium (K) and calcium (Ca) will not dissolve in hydrochloric acid or nitric acid, as they are less reactive than hydrogen and cannot displace hydrogen from these acids. Iron (Fe) and magnesium (Mg) will react with hydrochloric acid to form their respective chlorides and with nitric acid to form their respective nitrates.
Hydrogen is more reactive than potassium and calcium. When they are placed in hydrochloric or nitric acid, the hydrogen ions (H+) in the acid will not be displaced by potassium or calcium ions. Instead, the potassium and calcium ions will remain in the solution in their ionic form, and no reaction will occur.
However, iron and magnesium are more reactive than hydrogen. When they are placed in hydrochloric or nitric acid, the metal will react with the acid to form a salt and hydrogen gas.
For more question on acid click on
https://brainly.com/question/27915098
#SPJ11
please help which one is it

Answers
Answer:
17 g
Explanation:
if a chemist has 0.468 moles of c2h6o, how many moles of carbon will he get decomposing the sample?
Answers
If a chemist has 0.468 moles of \(C_{2}H_{6}O\), then 0.936 moles of carbon will be required for the decomposition of the sample.
Since \(C_{2}H_{6}O\) has the chemical formula \(C_{2}H_{6}O\), we can infer that it has two carbon atoms.
We must use the mole ratio of carbon to \(C_{2}H_{6}O\) to calculate the amount of carbon in 0.468 molecules of \(C_{2}H_{6}O\).
Each molecule of \(C_{2}H_{6}O\) contains 2 carbon atoms, as can be seen from the chemical formula. Consequently, the amount of carbon in moles is:
0.936 moles of C = 0.468 moles of \(C_{2}H_{6}O\) x (2 moles of C / 1 mole of \(C_{2}H_{6}O\)).
By dissolving 0.468 molecules of \(C_{2}H_{6}O\), the chemist will obtain 0.936 moles of carbon.
Therefore, if a chemist has 0.468 moles of \(C_{2}H_{6}O\), then 0.936 moles of carbon will be required for the decomposition of the sample.
Here is more information about moles: brainly.com/question/26416088
#SPJ4
(c) Is the value of the equilibrium constant, K, for the reaction greater than 1, or less than 1 ? Justify your answer.
Answers
The equilibrium constant, K, is a value that indicates the extent to which a reaction will proceed towards products at equilibrium.
If the value of K is greater than 1, it means that the products are favored at equilibrium, indicating that the reaction will proceed more towards products. On the other hand, if the value of K is less than 1, it means that the reactants are favored at equilibrium, indicating that the reaction will proceed more towards reactants.
To determine whether the value of K is greater than 1 or less than 1 for a specific reaction, we need to look at the balanced chemical equation for the reaction and calculate the equilibrium constant using the concentrations or pressures of the reactants and products at equilibrium. Without knowing the specific reaction, we cannot provide a definitive answer.
To determine if the value of the equilibrium constant, K, for the reaction is greater than 1 or less than 1, you need to consider the relationship between the concentrations of products and reactants at equilibrium. The equilibrium constant, K, is defined as the ratio of the concentrations of products to the concentrations of reactants, raised to the power of their respective stoichiometric coefficients.
If K > 1, it indicates that the concentration of products is greater than the concentration of reactants at equilibrium, meaning the reaction favors the formation of products.
If K < 1, it indicates that the concentration of reactants is greater than the concentration of products at equilibrium, meaning the reaction favors the formation of reactants.
In order to justify the value of K for the given reaction, you would need the equilibrium concentrations of the products and reactants or other information that allows you to determine the ratio of product concentrations to reactant concentrations at equilibrium.
Visit here to learn more about equilibrium constant:
brainly.com/question/10038290
#SPJ11
Define the term oxide
Answers
Answer: is the chemical compound that contains one oxygen atom and one other element in it chemical formular
Explanation:
Oxide - is a binary compound of oxygen with a more electropositive element or group
Hope this helps :))
Explain the difference between aliphatic hydrocarbons and aromatic hydrocarbons.
Answers
Answer:
Explanation:
Aliphatic compounds are those hydrocarbons that are the open chain compounds and also closed chains. Aromatic compounds are those who have only a closed chain structure. They can be saturated as well as unsaturated where the system can be open as well as closed chain.
4. Cell size is limited by the
(a) amount of cytoplasm.
(b) cell's ability to get rid of wastes.
(c) the size of the nucleus.
(d) the size of the plasma membrane.
Answers
Answer:
Maybe it's (a) amount of cytoplasm ...
hope its right
What is the difference between Chemistry and Alchemy?
Answers
Answer:
The difference between alchemy and modern chemistry is alchemy is based on a mystic, supernatural view of reality, whereas chemistry assumes reality is basically natural.
Explanation:
hope this helps!
Stacy made the following table to compare the functions of plant and animal structures, but she is missing a row title. Which of the following would best replace the X?
a table comparing the functions of different animal and plant structures. There is an unknown 'X' in the table. The skeleton in animals and the stem in plants provide support. The fur in animals and the waxy covering in plants provide protection. The ovaries in animals and the pistil in plants make 'X'
Answers
Answer:
female reproductive structures
A solution containing a mixture of lactic acid and lactate was found to have a pH of 4.26. Calculate the ratio of the lactate concentration to the lac
Answers
Given :
pH of solution of mixture of lactic acid and lactate is 4.26 .
To Find : \(log( \dfrac{[ \text{Conc. of lactate}]}{[\text{Conc . of lactic acid }]}) = 0.4\)
The ratio of the lactate concentration to the lactate .
Solution :
We know , pKa of lactic acid is :
pKa = 3.86 .
Now , the solution of lactic acid and lactate is a buffer solution .
So , pHof buffer acid is given by :
\(pH=pKa+log(\dfrac{[Salt]}{[Acid]})\)
Putting all given values :
\(log( \dfrac{[ \text{Conc. of lactate}]}{[\text{Conc . of lactic acid }]}) = 0.4\)
Taking anti log both the sides , we get :
\(\dfrac{[ \text{Conc. of lactate}]}{[\text{Conc . of lactic acid }]} = 2.51\)
Therefore , the ratio of the lactate concentration to the lactate is 2.51 .
Hence , this is the required solution .
500 ml of 3.31 M HCl is mixed with 56.1 g NaOH to produce water and table salt.
What mass of NaCl is produced?
ss
Answers
Answer:
m=1,48g
Explanation:
What variables are used to calculate speed
Answers
S /\ Speed
mph, km/h, ft/s
Answer:
Calculating speed, distance and time
We can use formulas to model real-life situations. For example, the formula for calculating speed is speed = distance ÷ time. It is possible to calculate the speed, distance or time if you have the other two values.
Explanation:
ENERGY ACCOUNTING: Per pyruvate: PYRUVATE OXIDATION • NADH produced: CITRIC ACID CYCLE • NADH produced: • FADH₂ produced: • ATP produced: ENERGY ACCOUNTING: Per glucose entering glycolysis: PYRUVATE OXIDATION • NADH produced: • NADH produced: FADH₂ produced: • • ATP produced: CITRIC ACID CYCLE
Answers
In terms of energy accounting, the breakdown of glucose and pyruvate through cellular respiration involves several steps. For pyruvate oxidation, the production of NADH is noted. In the citric acid cycle, NADH, FADH₂, and ATP are generated.
When considering glucose entering glycolysis, the energy accounting includes NADH, FADH₂, and ATP production.
Pyruvate oxidation occurs after glycolysis in the presence of oxygen. Each pyruvate molecule undergoes oxidation and is converted into Acetyl-CoA. During this process, one NADH molecule is produced per pyruvate.
The citric acid cycle, also known as the Krebs cycle, follows pyruvate oxidation. In this cycle, Acetyl-CoA enters and goes through a series of reactions. The products generated in the citric acid cycle include three NADH molecules, one FADH₂ molecule, and one ATP molecule per Acetyl-CoA.
As glucose produces two pyruvate molecules through glycolysis, the overall energy accounting for glucose entering glycolysis would be twice the values obtained for pyruvate oxidation.
Per pyruvate in Pyruvate Oxidation:
- NADH produced: 1
Per Acetyl-CoA in the Citric Acid Cycle:
- NADH produced: 3
- FADH₂ produced: 1
- ATP produced: 1
Per glucose entering Glycolysis:
- NADH produced: 2 (twice the value of pyruvate oxidation)
- FADH₂ produced: 0 (as FADH₂ is not directly generated in glycolysis)
- ATP produced: 2 (twice the value of pyruvate oxidation)
These values represent the energy accounting per pyruvate in pyruvate oxidation, per Acetyl-CoA in the citric acid cycle, and per glucose entering glycolysis. The production of NADH, FADH₂, and ATP molecules in these processes plays a crucial role in the generation of energy through cellular respiration.
Learn more about Glycolysis here :
https://brainly.com/question/14076989
#SPJ11