suppose that you obtain 1.0 g of isopentyl acetate from reacting 1.0 ml of acetic acid with 1.0 ml of isopentyl alcohol. calculate a percent yield.
Answers
The percentage yield of isopentyl acetate is 43956.04%.
The percentage yield is defined as the actual yield obtained from the reaction divided by the theoretical yield that should have been obtained under ideal conditions.
The equation used to determine percentage yield is given below:
Percentage yield = (Actual yield ÷ Theoretical yield) × 100
Given that 1.0 ml of acetic acid was reacted with 1.0 ml of isopentyl alcohol, the limiting reactant can be determined by carrying out a mole to mole comparison between the two reactants.
acetic acid (CH3COOH) + isopentyl alcohol (C5H12O) → isopentyl acetate (C7H14O2)
The balanced equation for the reaction shows that the mole ratio between acetic acid and isopentyl acetate is 1:1 and the mole ratio between isopentyl alcohol and isopentyl acetate is 1:1.
Therefore, the limiting reactant is the one that has the smallest number of moles.
The number of moles of acetic acid that reacted can be calculated as follows:
volume of acetic acid used = 1.0 ml = 0.001 L
density of acetic acid = 1.05 g/ml
molar mass of acetic acid = 60 g/mol
Number of moles of acetic acid = (volume × density) ÷ molarmass
= (0.001 L × 1.05 g/ml) ÷ 60 g/mol
= 0.0000175 mol
The number of moles of isopentyl alcohol that reacted can be calculated as follows:
volume of isopentyl alcohol used = 1.0 ml = 0.001 L
density of isopentyl alcohol = 0.81 g/ml
molar mass of isopentyl alcohol = 88 g/mol
Number of moles of isopentyl alcohol = (volume × density) ÷ molarmass
= (0.001 L × 0.81 g/ml) ÷ 88 g/mol
= 0.0000092 mol
Since acetic acid and isopentyl alcohol react in a 1:1 molar ratio, the number of moles of isopentyl acetate produced is equal to the number of moles of acetic acid that reacted, which is 0.0000175 mol.
The theoretical yield of isopentyl acetate can be calculated as follows:
number of moles of isopentyl acetate produced = 0.0000175
mol molar mass of isopentyl acetate = 130 g/mol
Theoretical yield of isopentyl acetate = number of moles × molar mass
= 0.0000175 mol × 130 g/mol
= 0.002275 g
The actual yield of isopentyl acetate obtained is given as 1.0 g.
The percentage yield can now be calculated as follows:
Percentage yield = (Actual yield ÷ Theoretical yield) × 100
= (1.0 g ÷ 0.002275 g) × 100= 43956.04%
Therefore, the percentage yield of isopentyl acetate is 43956.04%.
Learn more about the percentage yield:
brainly.com/question/30400363
#SPJ11
Related Questions
what happens when hydrochloric acid (hcl) is added to a carbonate salt? observation reaction products
Answers
When hydrochloric acid (HCl) is added to a carbonate salt, a chemical reaction occurs, producing carbon dioxide (CO2) gas, water (H2O), and a chloride salt. This reaction is called an acid-carbonate reaction. The key observation during this reaction is the effervescence or bubbling due to the release of carbon dioxide gas.
In this process, the HCl donates a hydrogen ion (H+) that reacts with the carbonate ion (CO3 2-) present in the carbonate salt. The reaction results in the formation of carbonic acid (H2CO3), which then breaks down into water and carbon dioxide gas.
The remaining part of the carbonate salt combines with the chloride ion (Cl-) from the hydrochloric acid to form the corresponding chloride salt.
The general equation for this reaction can be written as:
2HCl(aq) + M2CO3(s) → 2MCl(aq) + H2O(l) + CO2(g)
Here, M represents the metal cation of the carbonate salt, such as sodium (Na), calcium (Ca), or potassium (K).
An observable sign of this reaction is the effervescence or bubbling due to the release of carbon dioxide gas. The solution may also become clearer as the carbonate salt dissolves and reacts with the acid.
For more such questions on hydrochloric acid, click on:
https://brainly.com/question/3229358
#SPJ11
Use the solubility curves to answer the questions below
1) a. What is the solubility of potassium chloride at 80•c?
b. What is the solubility of potassium chloride at 40•c?
2) What mass of potassium chloride would crystallise out of solution if a saturated solution in 100g of water was cooled from 80•c to 40•c?

Answers
1) To determine the solubility of potassium chloride at different temperatures, we can refer to a solubility curve for potassium chloride. Unfortunately, since the solubility curve is not provided, I cannot give you the exact solubility values at 80°C and 40°C. Solubility is typically given in grams of solute per 100 grams of solvent (usually water) at a specific temperature.
2) To calculate the mass of potassium chloride that would crystallize out of solution, we need to determine the difference in solubility between 80°C and 40°C. Let's assume that at 80°C, the solubility of potassium chloride is 50 g/100 g of water, and at 40°C, the solubility is 30 g/100 g of water.
The initial amount of potassium chloride in the solution is 50 g (saturated solution in 100 g of water at 80°C). At 40°C, the solubility decreases to 30 g/100 g of water.
The amount of potassium chloride that crystallizes out can be calculated by subtracting the final solubility from the initial amount:
50 g - 30 g = 20 g
Therefore, 20 grams of potassium chloride would crystallize out of the solution when cooled from 80°C to 40°C.
Kindly Heart and 5 Star this answer, thanks!True or False: Energy is conserved as a transmembrane pH gradient
Answers
False. While a transmembrane pH gradient does represent a form of potential energy, it is not a conserved form of energy.
The movement of ions across a membrane to establish a pH gradient involves energy expenditure, typically in the form of ATP hydrolysis. In biological systems, this energy is used to drive a range of processes, from nutrient uptake to the synthesis of ATP itself. Ultimately, however, the energy contained in the pH gradient is dissipated when ions move back across the membrane, either through passive diffusion or active transport. This movement of ions is accompanied by the release of heat and the loss of energy, meaning that the initial potential energy represented by the pH gradient is not conserved. Overall, while the transmembrane pH gradient is an important component of many biological systems, it is not a conserved form of energy.
Learn more about ATP :
https://brainly.com/question/14637256
#SPJ11
When burning 180g of glucose in the presence of 192g of oxygen, water and carbon dioxide are produced. If 108g of water is produced, how many grams of carbon dioxide are produced?
Answers
The mass of carbon dioxide in this is 264 g according to the given data.
What is molar mass?The molar mass is basically the total mass in grams of the atoms that make up a molecule per mole. Molar mass is measured in grams per mole.
Combustion is the process of burning glucose in the presence of oxygen.
The balanced reaction is
\(C_6H_1_2O_6+6O_2---- > 6CO_2+6H_2O\)
As a result, each mole of glucose requires six moles of oxygen molecules.
Glucose has a molar mass of 180g per mole.
The present moles of glucose = mass / molar mass = 180 / 180 = 1 moleThe present moles of oxygen = mass / molar mass = 192 / 32 = 6 molesAs a result, the reaction will be completed, with one mole of glucose reacting with six moles of oxygen to produce six moles of Carbon dioxide and six moles of water.
Carbon dioxide produced mass = moles X molar mass = 6 x 44 = 264 g
Thus, 264g will be the mass of the carbon dioxide produced.
For more details regarding molar mass, visit:
https://brainly.com/question/22997914
#SPJ1
Which statements are true of the reaction below? 2Na(s) + Cl2(g) ---> 2NaCl(s)

A.
Na(s) is a reactant.

B.
NaCl is a product.

C.
NaCl is a liquid.

D.
Cl2 is a solid.
Answers
Answer:
A and B are both true
Explanation:
Na is REACTING with Cl2 to PRODUCE NaCl
in the late 18th century, lazzaro spallanzani boiled broths for long periods of time and sealed the flasks by melting their glass necks closed. based on the results of his experiments, he concluded that
Answers
Lazzaro Spallanzani concluded that boiling the broth killed any potential living organisms and that air was not necessary for life, based on the results of his experiments boiling broths and sealing the flasks.
Lazzaro Spallanzani was an Italian scientist who lived in the late 18th century. In his experiments, he boiled broths for extended periods of time and then sealed the flasks by melting their glass necks closed. By doing this, he aimed to determine the conditions necessary for life to exist.
The results of his experiments showed that boiling the broth killed any potential living organisms and that the broth did not contain any new microorganisms after being sealed and boiled. This led Spallanzani to conclude that air was not necessary for life and that life could not arise spontaneously from non-living matter.
These findings were crucial in disproving the then-prevailing idea of spontaneous generation, which was the belief that life could arise spontaneously from non-living matter. Spallanzani's experiments paved the way for further advancements in microbiology and the understanding of the origins of life.
Learn more about Spallanzani's experiments here:
https://brainly.com/question/17385986
#SPJ4
what is the mass of electron
Answers
Explanation:
The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 1/1,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom.
Answer: The Mass of an electron is 9.1093837 x 10^-31 kgs
Explanation:
What is the volume of a weather balloon that has 39 grams of helium with a density 0.017 g/mL?
Answers
Answer:
The answer is 235.29 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \\ \)
From the question.
density = 0.017 g/mL
mass = 4 g
We have
\(volume = \frac{4}{0.017} \\ = 235.2941176...\)
We have the final answer as
235.29 mLHope this helps you
Tpr (temperature, pressure, and relief) valves must be set to discharge at a temperature no higher than ________
Answers
Tpr (temperature, pressure, and relief) valves must be set to discharge at a temperature no higher than 210°F.
What is temperature?Temperature can simply be defined as the degree of hotness or coldness of a body.
The instrument used for measuring temperature is thermometer
So therefore, Tpr (temperature, pressure, and relief) valves must be set to discharge at a temperature no higher than 210°F
Learn more about temperature:
https://brainly.com/question/25677592
#SPJ1
Which group has the highest ionization energies? Explain why.
Answers
Answer:
nobel elements (gr. 18) because they are fully stable due to octet complete--------------------------------------------------
A rocket can be powered by the reaction between dinitrogen tetroxide and hydrazine:
20a
An engineer designed the rocket to hold 1. 35 kg N2O4 and excess N2H4. How much N2 would be produced according to the engineer's design? Enter your answer in scientific notation.
Answers
Expressing this answer in scientific notation, the amount of N2 produced according to the engineer's design would be approximately 1.467 x 10^1 mol.
To determine the amount of N2 produced in the reaction between dinitrogen tetroxide (N2O4) and excess hydrazine (N2H4), we need to consider the stoichiometry of the reaction.
The balanced equation for the reaction is:
N2H4 + N2O4 → N2 + 2H2O
According to the stoichiometry of the reaction, for every one mole of N2H4, one mole of N2 is produced. The molar mass of N2H4 is approximately 32.05 g/mol.
Given that the rocket is designed to hold 1.35 kg (1350 g) of N2O4, we can calculate the moles of N2H4 required:
Moles of N2H4 = Mass of N2O4 / Molar mass of N2O4
Moles of N2H4 = 1350 g / 92.01 g/mol ≈ 14.67 mol
Since the stoichiometry is 1:1, the amount of N2 produced will be equal to the moles of N2H4:
Moles of N2 produced = Moles of N2H4 ≈ 14.67 mol
Expressing this answer in scientific notation, the amount of N2 produced according to the engineer's design would be approximately 1.467 x 10^1 mol.
For more question on scientific notation
https://brainly.com/question/30406782
#SPJ8
Which statements are true about light waves? (Select all that apply.)
Light waves are sun waves.
Light waves are frequency waves.
Light waves are electromagnetic waves.
Light waves are transverse waves.
PLEASE SELECT MORE THAN ONE
WILL MARK BRAINLY :D✌️
Answers
Answer:
only transverse and electromagnetic waves
Explanation:
Light waves are electromagnetic waves.Electromagnetic waves or EM waves exist waves that are created as a consequence of vibrations between an electric field and a magnetic field.
What are electromagnetic waves?In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, propagating via space, and having electromagnetic radiant energy. It contains radio waves, microwaves, infrared, (visible) light, ultraviolet, X-rays, and gamma rays. All of these waves form parts of the electromagnetic spectrum.
Light waves are electromagnetic waves.Electromagnetic waves or EM waves exist waves that are created as a consequence of vibrations between an electric field and a magnetic field.
Learn more about electromagnetic waves refer to:
https://brainly.com/question/26960785
#SPJ2
100 pOiNtS
Explain why the vapor pressure of a solution differs from that of the pure solvent
Explain why the freezing point of a solution differs from that of the pure solvent
Explain why the boiling point of a solution differs from that of the pure solvent
Answers
Answer:
Answer one:
Solute particles block some of the ability of liquid particles to evaporate. We can then conclude, solutions of solid solutes typically have a lower vapor pressure than pure solvents.
Answer two:
Solutions freezing points are lower than of the pure solvent or solute because freezing (becoming a solid) creates order and decreases entropy.
Answer three:
The presence of solute particles decreases the vapor pressure of the liquid solvent, this means a higher temperature is needed to reach the boiling point.
Explanation:
Have a great rest of your day
#TheWizzer

which of the following is a product in the chemical equation N + O2 = NO2
1. NO2
2. N
3. O
4. O2
Answers
Could someone help me with this pls i need it asap chimestry
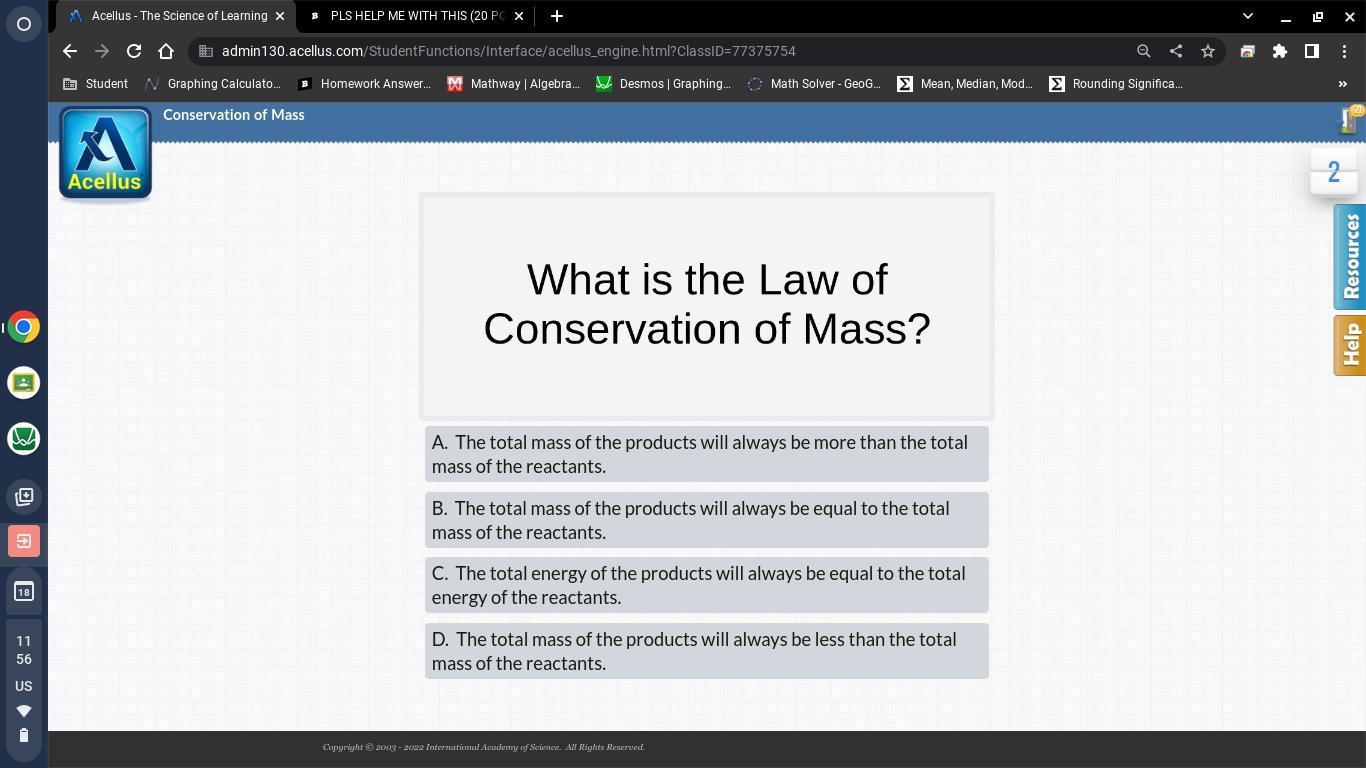
Answers
Answer:
b
Explanation:
the mass stays the same, it is only rearanged
Answer: B
Explanation: Law of Conservation of Mass states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass cannot change, so quantity can neither be added nor be removed.
1. calculate the ph of a buffer solution made from equal amounts of 0.30 m hydrofluoric acid and 0.70 m sodium fluoride. ka=7.1×10−4
Answers
A buffer solution can resist a change in pH even when a strong acid or a strong base is added to it. A buffer solution is made up of a weak acid and its conjugate base, or a weak base and its conjugate acid.A hydrofluoric acid-sodium fluoride buffer solution can be made from hydrofluoric acid and sodium fluoride.
The buffer solution can be calculated as follows: Hydrofluoric acid is a weak acid, with a Ka of 7.1 × 10−4.Moles of Hydrofluoric acid (HF) = 0.30 × VolumefHF = [HF]/V = 0.30 mMoles of sodium fluoride (NaF) = 0.70 × VolumefNaF = [NaF]/V = 0.70 mMoles of Hydrogen Fluoride (H+) = Molarity × Volume = 0.30 × VolumepH = pKa + log ([A-]/[HA])Ka = [H+][A-]/[HA]7.1 × 10−4 = [H+][NaF]/[HF][H+] = 5.3 × 10−4[Naf]/[HF] = 7/3log [NaF]/[HF] = log (7/3) = 0.851pH = pKa + log ([A-]/[HA])pH = 3.86 + 0.851 = 4.71Therefore, the pH of a buffer solution made from equal amounts of 0.30 M hydrofluoric acid and 0.70 M sodium fluoride is 4.71.
For more information on buffer solution visit:
brainly.com/question/31428923
#SPJ11
Convert 3.99/gallon to dollars per liter
Answers
Explanation:
Gas prices in the U.S. are given by the gallon; to convert them to liters, you'll divide the price by the number of liters in a gallon, 3.78541. So if gas costs $3.50 per gallon, you have $3.50 ÷ 3.78541 = $0.92460 per liter, which would typically be rounded to $0.92 per liter.
if a reaction vessel initially contains an n2o4 concentration of 0.0550 m at 500 k , what are the equilibrium concentrations of n2o4 and no2 at 500 k ? express your answers in moles per liter to three significant figures separated by a comma.
Answers
The equilibrium concentrations of N₂O₄ and NO₂ at 500 K are 0.0287 mol/L and 0.526 mol/L, respectively.
The balanced equation for the reaction is:
N₂O₄ (g) <==> 2NO₂(g)
At equilibrium, the equilibrium constant (Kc) is given as 0.513, and the initial concentration of N₂O₄ is 0.0550 M. Let x be the equilibrium concentration of NO₂.
Then, the equilibrium concentrations of N₂O₄ and NO₂ can be calculated as follows:
Kc = [NO₂]² / [N₂O₄]
0.513 = (2x)^2 / (0.0550 - x)
0.513(0.0550 - x) = 4x²
0.028215 - 0.513x = 4x²
4x² + 0.513x - 0.028215 = 0
Solving this quadratic equation, we get:
x = 0.263 mol/L
Therefore, the equilibrium concentrations of N₂O₄ and NO₂ are:
[N₂O₄] = (0.0550 - x) = 0.0550 - 0.263 = 0.0287 mol/L
[NO₂] = 2x = 0.526 mol/L
So, the equilibrium concentrations of N₂O₄ and NO₂ at 500 K are 0.0287 mol/L and 0.526 mol/L, respectively.
Learn more about N₂O₄ here: brainly.com/question/20464123
#SPJ4
Complete question:
For the reaction N₂O₄ (g) <==> 2NO₂ (g) Kc =0.513 at 500 K.If a reaction vessel initially contains an N₂O₄ concentration of 0.0550 M at 500 K, what are the equilibrium concentrations of N₂O₄and NO₂ at 500 K? Express your answers in moles per liter to three significant figures separated by a comma.
Which undergoes dehydration using concentrated h 2so 4 faster, cyclopentanol or 1-methylcyclopentanol? explain briefly.
Answers
1-Methylcyclopentanol undergoes dehydration faster than cyclopentanol when treated with concentrated sulfuric acid (H₂SO₄). This is due to the presence of the methyl group (CH₃) attached to the cyclopentanol molecule.
Alcohols are dehydrated when a water molecule (H₂O) is taken out of the alcohol molecule. To speed up the reaction rate in this procedure, an acid catalyst such concentrated sulfuric acid is frequently utilized.
In comparison to cyclopentene, 1-methylcyclopentanol's methyl group accelerates the rate of dehydration. This is so because the methyl group, which donates electron density to the nearby carbon atom (alpha carbon) in the molecule, is an electron-donating group. The acid catalyst is more likely to attack the alpha carbon due to its higher electron density.
Since the protonation of the 1-methylcyclopentanol's alpha carbon by the acid catalyst proceeds more quickly as a result, a more stable carbocation intermediate is created. This makes it easier for a water molecule to be lost later and for the equivalent alkene product to develop.
Cyclopentanol, on the other hand, is devoid of the electron-donating methyl group and has a reduced electron density on the alpha carbon. As a result, compared to 1-methylcyclopentanol, the protonation step takes longer, and the dehydration reaction as a whole is less effective.
Therefore, when exposed to strong sulfuric acid, 1-methylcyclopentanol dehydrates more quickly than cyclopentanol due to the presence of the methyl group.
To know more about cyclopentene:
https://brainly.com/question/33422683
#SPJ4
The concentration of which ion is increased when LIOH is dissolved in water
Answers
Answer:OH^- ion
Explanation: When LiOH added in water then LiOH dissociated in Li+ and OH- . Water is a composition of H+ and OH- ions hence concentration of OH- ions will be increased after adding LiOH.
how many milliliters of acid are required to reach the equivalence point for the titration of 50.0 ml of 1.0 mnaoh with 1.0 mhcl ?
Answers
50.0 mL of 1.0 M HCl are required to reach the equivalence point in the titration of 50.0 mL of 1.0 M NaOH.
To determine how many milliliters of 1.0 M HCl are required to reach the equivalence point in the titration of 50.0 mL of 1.0 M NaOH, we can use the following steps:
1. Write the balanced chemical equation for the reaction:
\(NaOH + HCl --> NaCl + H_2O\)
2. Determine the moles of NaOH:
Moles of NaOH = volume (L) × concentration (M)
Moles of NaOH = 0.050 L × 1.0 M = 0.050 moles (Note: 50.0 mL = 0.050 L)
3. Determine the moles of HCl needed for the reaction:
From the balanced equation, the ratio of NaOH to HCl is 1:1. So, the moles of HCl needed is the same as the moles of NaOH. Moles of HCl = 0.050 moles
4. Calculate the volume of HCl required to reach the equivalence point:
Volume of HCl (L) = moles of HCl / concentration of HCl
Volume of HCl (L) = 0.050 moles / 1.0 M = 0.050 L
5. Convert the volume of HCl to milliliters:
Volume of HCl (mL) = 0.050 L × 1000 mL/L = 50.0 mL
To learn more about titration click here https://brainly.com/question/31271061
#SPJ11
write down the two chemical reaction which are carried out by the catalyst.
Answers
A grocer carefully lifts a 100 N crate of apples a distance of 1.5 m to a shelf in 2.5 seconds. What is his power output?
Answers
The grocer's power output is 60 Watts. Power is measured in Watts, which represents the rate of energy transfer or work done per unit time.
Power is defined as the rate at which work is done or energy is transferred. It can be calculated using the formula: Power = Work / Time.
In this case, the work done by the grocer is equal to the force applied multiplied by the distance moved. The force applied is 100 N and the distance moved is 1.5 m, so the work done is:
Work = Force * Distance
Work = 100 N * 1.5 m
Work = 150 Joules
The time taken to perform the work is 2.5 seconds. Now we can calculate the power output:
Power = Work / Time
Power = 150 Joules / 2.5 seconds
Power = 60 Watts
Therefore, the grocer's power output is 60 Watts. Power is measured in Watts, which represents the rate of energy transfer or work done per unit time. It indicates how quickly the grocer is able to lift the crate of apples to the shelf.
For more questions on force, click on:
https://brainly.com/question/12785175
#SPJ8
Who was the first person to introduce the concept of atomic mass?
Answers
according to Bohr atomic model
Answers
Answer:
A small positively charged nucleus surrounded by revolving negatively charged electrons in fixed orbits
Compound Molar mass (g/mol)
NaCN
49.0
65.0
40.0
58.4
NaN3
NaOH
NaCl
Based on the information in the table, which of the following compounds
contains the greatest percentage of sodium by mass?
Answers
Answer:
Calculating the molar mass of each compound as well as the mass of the sodium in each compound will help us identify which compound has the highest mass percentage of sodium. After that, we can determine the salt content in mass.
Molar mass of NaCN = 49.0 g/mol
Mass of Na in NaCN = 23.0 g/mol
Percentage of Na by mass in NaCN = (23.0 g/mol / 49.0 g/mol) x 100% = 46.9%
Molar mass of NaN3 = 65.0 g/mol
Mass of Na in NaN3 = 23.0 g/mol
Percentage of Na by mass in NaN3 = (23.0 g/mol / 65.0 g/mol) x 100% = 35.4%
Molar mass of NaOH = 40.0 g/mol
Mass of Na in NaOH = 23.0 g/mol
Percentage of Na by mass in NaOH = (23.0 g/mol / 40.0 g/mol) x 100% = 57.5%
Molar mass of NaCl = 58.4 g/mol
Mass of Na in NaCl = 23.0 g/mol
Percentage of Na by mass in NaCl = (23.0 g/mol / 58.4 g/mol) x 100% = 39.4%
Therefore, NaOH contains the greatest percentage of sodium by mass, at 57.5%.
Based on the masses that react, we have 0.5 mol of \(NaOH\) and 0.185 mol of FeCl₃, which react to form 0.185 mol of Fe(OH)₃.
To calculate the amount (mol) of each compound based on the masses that react, you first need to use the given molar masses to convert the mass of each compound to moles. This can be done using the formula:
moles = mass (in grams) / molar mass (in grams/mol)
For example, if we have 20 grams of NaOH, we can calculate the number of moles as:
moles\(NaOH\) = 20 g / 40.00 g/mol = 0.5 mol
Similarly, if we have 30 grams of \(FeCl₃,\) we can calculate the number of moles as:
moles FeCl₃ = 30 g / 162.21 g/mol = 0.185 mol
Therefore, we have 0.5 mol of NaOH and 0.185 mol of FeCl₃ reacting with each other. The balanced chemical equation for the reaction is:
\(3 NaOH + FeCl₃ → Fe(OH)₃ + 3 NaCl\)
From the equation, we can see that 3 moles of NaOH react with 1 mole of FeCl₃ to produce 1 mole of Fe(OH)₃ and 3 moles of NaCl. Since we have excess NaOH in this case, we can use the amount of FeCl₃ to determine the limiting reactant and the amount of product formed.
Since we have 0.185 mol of FeCl₃ and it reacts with 3 moles of NaOH, the amount of NaOH required for complete reaction would be:
moles \(NaOH required = 0.185 mol FeCl₃ × (3 mol NaOH / 1 mol FeCl₃) = 0.555 mol\)
Since we have 0.5 mol of NaOH, it is the limiting reactant and only 0.185 mol of FeCl₃ will react to form the product. The amount of Fe(OH)₃ formed can be calculated as:
\(moles EditCopy equationRemove formed = 0.185 mol FeCl₃ × (1 mol Fe(OH)₃ / 1 mol FeCl₃) = 0.185 mol\)
Therefore, we have 0.5 mol of\(NaOH\)and 0.185 mol of FeCl₃, which react to form 0.185 mol of Fe(OH)₃.
Learn more about molar mass on:
https://brainly.com/question/31545539
#SPJ6
How many liters of hydrogen are obtained from the reaction of 4.00 g sodium with excess water, at STP?
Answers
Answer:
V = 1.95 L.
Explanation:
Hell there!
In this case, according to the following reaction between sodium metal and water:
\(2Na+2H_2O\rightarrow 2NaOH+H_2\)
We can realize that the moles of hydrogen can be calculated by using the initial mass of sodium, its atomic mass (23.0 g/mol) and the 2:1 mole ratio of sodium to hydrogen to obtain:
\(4.00gNa*\frac{1molNa}{23.0gNa} *\frac{1molH_2}{2molNa}=0.0870molH_2\)
Finally, we calculate the volume of hydrogen by using the ideal gas equation whereas the pressure is 1 atm and the temperature 273.15 K according to the STP conditions:
\(PV=nRT\\\\V=\frac{nRT}{P}\\\\V=\frac{0.0870mol*0.08206\frac{atm*L}{mol*K}*273.15 K}{1 atm}\\\\V=1.95L\)
Regards!
(a) compute the repeat unit molecular weight of polypropylene. (b) compute the number-average molecular weight for a polypropylene for which the degree of polymerization is 25000.
Answers
The repeat unit molecular weight of polypropylene is 42.08 g/mol . For polypropylene, each repeat unit has three carbons and six hydrogens.
Thus, m= 3(AC) + 6(AH)
= (3)(12.01 g/mol) + (6)(1.008 g/mol)
= 42.08 g/mol
Now computation of the number-average molecular weight.
Since the degree of polymerization (DP) is 25,000,
number-average molecular weight = (DP)m
= (25,000)(42.08 g/mol)
= 1,052,000 g/m
What is polypropylene?It is a thermoplastic polymer made from propylene units.
What is polymerization?Polymerization is the process of forming long chainlike compounds reacting monomer molecules together in a chemical reaction.
What is molecular weight?It is the sum total of weight of all the atoms present in a substance.
To know more about polymerization, visit:
https://brainly.com/question/8752840
#SPJ4
pls help!!!!!!!!!!!!!!

Answers
The given reaction is the calcium carbide reaction of calcium hydroxide with water to produce acetylene.
How much C2H2 AND Ca(OH)2 was produced?Chemical equations must obey the law of conservation of mass and the law of constant proportions. That is, the reactant and product sides of the equation must contain the same number of atoms of each element or compound.The given reaction is the calcium carbide reaction of calcium hydroxide with water to produce acetylene.The balanced equation is CaC2+2H2OCa(OH)2+C2H2.
Molar mass of acetylene, C2H2 = 2x12+2x1 = 26 g/mol
we get 64 g of CaC2 becomes 26 g of C2H2. 5.0g CaC2..
Molecular weight of Ca(OH)2=1(atomic weight of Ca)+2(atomic weight of O)+2(atomic weight of H)=40+2(16)+2(1)=40+32+2=74.
For more information on Molecular weight, see:
https://brainly.com/question/27988184
#SPJ1
I WILL GIVE YOU BRAINLY PLSS!!! :(
Budding is a form of ______________ reproduction in which a new ______________
develops from a bud or outgrowth due to _________ division. The new organism remains
attached as it grows, separating from the ___________________ only when it is mature.
3. Vegetative reproduction is a form of _____________ reproduction in ___________ .
It is a process by which new organisms are produced without _______________ or
_______________ . It can occur naturally or be induced by ______________________ .
Answers
Answer:
Budding is a type of asexual reproduction in which a new organism develops from an outgrowth or bud due to cell division at one particular site.
Explanation:
Answer:
Budding is a type of asexual reproduction in which a new organism develops from an outgrowth or bud due to cell division at one particular site.
Explanation: