The best scientific theories not only explain and organize known facts but also
O a. generate new predictions.
O b. use historical perspectives.
O c. establish new facts.
O d. discredit other theories.
Answers
The best scientific theories not only explain and organize known facts but also (a) generate new prediction.
A scientific theory is made by performing various experiments to verify the observation made. A scientific theory is made by observing something, making hypothesis and testing that hypothesis using experiments and then coming to a conclusion. If the hypothesis made is proved wrong then a new hypothesis is made. And the process is repeated again.
A theory that is accepted now can be proved wrong in the near future. Or one can say that theory not necessarily be applicable in every situation.
The hypothesis that are made are nothing but generation of new predictions.
To know more about scientific theories here
https://brainly.com/question/2375277?
#SPJ1
Related Questions
Acetaldehyde has the chemical formula C₂H4O. Calculate the number of moles and C₂H₂O molecules in 475 g of acetaldehyde. HINT (a) moles moles (b) molecules molecules
Answers
RFM of C2 H2 O = (12x2)+2+16=42
Mass = 475
475/42=
11.31 moles
help me please. Can you please explain to me how to solve them? you can also send me a photo if that's better for you. thanks alot

Answers
Refer to the attachment

how would the value of the atomic mass of the metal calculated be affected if the hot metal sample cooled off before it was transferred to the water in the calorimeter? would it be too high or too low?
Answers
The value of the atomic mass of the metal calculated would be too high if the hot metal sample cooled off before it was transferred to the water in the calorimeter.
How is atomic mass calculated?The atomic mass of an element is defined as the mass of an atom of an element in atomic mass units (amu). One atomic mass unit is defined as 1/12th of the mass of an atom of carbon-12.
The atomic mass of an element can be calculated using the following formula:
Atomic mass = (mass of isotope 1 × % abundance of isotope 1) + (mass of isotope 2 × % abundance of isotope 2) + (mass of isotope 3 × % abundance of isotope 3) + ...
If the hot metal sample cooled off before it was transferred to the water in the calorimeter, the temperature of the sample would have decreased. The decrease in temperature would result in a decrease in the thermal energy of the sample. Consequently, the amount of heat absorbed by the water in the calorimeter would decrease, leading to a lower value of the heat capacity of the metal.
Since the heat capacity is directly proportional to the mass of the sample, a lower value of the heat capacity would lead to a higher value of the atomic mass of the metal calculated. Therefore, the value of the atomic mass of the metal calculated would be too high if the hot metal sample cooled off before it was transferred to the water in the calorimeter.
Learn more about atomic mass here: https://brainly.com/question/3187640.
#SPJ11
A high level of ________________ in your urine could show that you have diabetes.
Answers
Answer:
ketones.
Explanation:
If you have a high level of ketones in your urine, it usually means you're not getting enough insulin, which is a sign of Diabetes.
(I got this Information from the "Ketones in Urine" article from MedlinePlus.)
What two effects a soluble impurity usually has on the melting point of a compound.
Answers
A soluble impurity usually has two effects on the melting point of a compound: an increase in melting point range and a decrease in melting point.
In chemistry, there are two types of impurities: soluble and insoluble.
Soluble impurities are those that dissolve in water quickly and also have a significant impact on the chemical properties of a compound such as a significant increase or decrease in the melting point and boiling point.
For example, salt, milk, and sugar compounds are soluble impurities.
Similarly, insoluble impurities remain undissolved when mixed in water and are also known as suspended impurities.
Examples of insoluble impurities are sand, oil, chalk, rocks, pebbles, etc.
If you need to learn more about compounds click here:
https://brainly.com/question/26487468
#SPJ4
Implementing a new system into an organization module by module is called a ____________.
Answers
Implementing a new system into an organization module by module is called a Phased Approach.
The "phased approach," as the name implies, is a project planning strategy in which anything new, such as a software solution, is introduced in stages rather than all at once.
Rather than installing and rolling out new software across an entire organization at the same time, older systems/methodologies are gradually replaced. Phased implementation necessitates extensive project planning to determine which software functions should go live first and for which departments.
This process allows you to keep the most critical departments operational while others transition to the new system. However, phasing may not be necessary for every company or project. As software and technology become more complex, however, the phased approach reduces risk and often makes the most business sense.
Find more on phase related questions at : brainly.com/question/28162704
#SPJ4
An analyst was preparing standard solutions. While transferring the solid analyte to the volumetric flask, some amount of the solid stuck to the weigh paper and was not transferred. The measured concentrations came out a bit low for this solution. This is an example of:
Answers
The phenomenon described in the given question, where some amount of the solid stuck to the weigh paper and was not transferred, is an example of a volumetric error in the preparation of standard solutions.
A standard solution is a solution with a precisely known concentration of an element or a substance. They are prepared to a specific concentration by dissolving a known quantity of a pure substance in a particular solvent in a volumetric flask.
Volumetric errors are a type of systematic error that occurs when preparing standard solutions. They occur due to an error in the volume of solution added or when some of the solute gets lost during the transfer process. As a result, the measured concentration comes out a bit low for this solution.
To avoid such errors, it is essential to pay close attention to the precise amount of the analyte used and be careful while transferring it to the volumetric flask. Any loss of the solid during the transfer process will result in an incorrect concentration of the solution and can lead to incorrect results.
Thus, the correct answers is volumetric error.
To learn more about concentration :
https://brainly.com/question/17206790
#SPJ11
- The pit of an aqueous solution of NaOH is 12.9. What is the molarity of the solution?
Answers
Answer: The molarity of solution is 0.08 M
Explanation:
pH is the negative logarithm of hydrogen ion concentration. It tells abpout the acidity or basicity of a solution.
\(pH=-log[H^+]\)
also pH+pOH=14
given : pH of NaOH = 12.9
Thus pOH = (14-12.9) = 1.1
Thus \([OH^-]=10^{-1.1}=0.08M\)
As \(NaOH\rightarrow Na^++OH^-\)
As 1 mole of \(OH^-\) is produced by = 1 mole of NaOH
Thus 0.08 moles of \(OH^-\) are produced by = \(\frac{1}{1}\times 0.08=0.08\) moles of NaOH
The molarity of solution is 0.08 M
Compared to a 1-kg block of solid iron, a 2-kg block of solid iron has twice as much.
Answers
Compared to a 1-kg block of solid iron, a 2-kg block of solid iron has twice the mass.
mass is the amount of matter present in a particle.
unit of mass is kg.
weight is the force acting on the particle due to the gravity of the earth.
unit of weight is N.
let 1 kg block has mass x
then 2 kg block will have 2x mass
we can say that it has twice mass.
Compared to a 1-kg block of solid iron, a 2-kg block of solid iron has twice mass
For more information on mass click on the link below:
https://brainly.com/question/19385703
#SPJ4
according to molecular orbital theory, how many pi-antibonding molecular orbitals are there for benzene
Answers
According to molecular orbital theory, there are three pi-antibonding molecular orbitals in benzene. These molecular orbitals are formed by the out-of-phase combination of pi orbitals of adjacent carbon atoms.
In benzene, there are six pi orbitals that form a delocalized pi system above and below the plane of the molecule. These pi orbitals interact with each other to form three bonding pi molecular orbitals and three antibonding pi molecular orbitals.
The bonding pi molecular orbitals are lower in energy and are occupied by the six pi electrons, while the three higher-energy antibonding pi molecular orbitals are unoccupied.
Since pi-antibonding orbitals are destabilizing and lead to weaker bonds, the presence of these three pi-antibonding molecular orbitals in benzene suggests that the pi bonds in the molecule are weaker than the sigma bonds.
For more questions like Benzene click the link below:
https://brainly.com/question/7284916
#SPJ11
What causes alkaline phosphatase levels to be high.
Answers
Answer:
ALP is an enzyme found throughout the body, but it is mostly found in the liver, bones, kidneys, and digestive system. When the liver is damaged, ALP may leak into the bloodstream. High levels of ALP can indicate liver disease or bone disorders
Suppose that 1500 kJ of energy were transferred to water at 20.0°C. What mass of water could be brought to the boiling point? Heat capacity (c) for liquid water is 4.18 J/g C
Answers
That 1500 kJ of energy were transferred to water at 20.0°C. Heat capacity (c) for liquid water is 4.18 J/g C. mass of water could be brought to the boiling point is 4485 g.
given that :
heat energy = 1500 kJ
heat capacity , c = 4.18 J/g °C
initial temperature = 20.0°C
boiling of water ,final temperature = 100 °C
Q = mcΔT
m = Q / (cΔT)
m = 1500 / ( 4.18 × ( 100 °C - 20 °C )
m = 1500 / 334.4
m = 4.485 kg = 4485 g
Thus, That 1500 kJ of energy were transferred to water at 20.0°C. Heat capacity (c) for liquid water is 4.18 J/g C. mass of water could be brought to the boiling point is 4485 g.
To learn more about heat capacity here
https://brainly.com/question/17058254
#SPJ1
A solution of 0.220 g of KIO3 is prepared in 50 ml volumetric flask. 2.00 mL of this solution is pipetted into a beaker with 8.00 ml distilled water, to give 10 ml of diluted KIO3. To this, 10.00 mL of starch sulfite was added. What would be the initial concentration of IO3- in this final solution
Answers
Answer:
0.00044 g/mL
Explanation:
First, 0.220 g of KIO₃ are dissolved in 50 mL, so the concentration at this point is:
0.220 g / 50 mL = 0.0044 g/mLThen 2.00 mL of this solution are added to 8.00 mL of water (final volume = 10.00 mL). We calculate the concentration of the resulting solution:
0.0044 g/mL * 2.00mL / 10.00mL = 0.00088 g/mLFinally, to this 10 mL solution, another 10.00 mL were added. Thus the KIO₃ (or IO₃⁻) concentration is:
0.00088 g/mL * 10.00mL / 20.00mL = 0.00044 g/mLA plane flies from NY to LA at a constant speed of 800 km/hr. How long will it take the plane to fly the 4200 km? 5
Answers
As the plane plane flies at a constant speed of 800 km/hr the plane will take 5 hours 25 minutes to fly distance of 4200 km.
What is speed?Speed is defined as the rate of change of distance with time. It has the dimension of distance by time. Thus, the SI unit of speed is given as the combination of the basic unit of distance and the basic unit of Time. Thus, the SI unit of speed is metre per second. In everyday life, kilometre per hour or in countries like US and UK miles per hour are used as the unit of speed.
The SI unit of speed can be derived from the formula of velocity. Basically, velocity is the vector equivalent of speed. Mathematically, velocity is given as the ratio of displacement to the time taken.So, 800 km is traveled in 1 hour so 4200 km will be traveled 4200/800=5.25 which is 5 hours and 25 minutes.
Thus, the plane will take 5 hours 25 minutes to fly distance of 4200 km.
Learn more about speed,here:
https://brainly.com/question/7359669
#SPJ5
What can scientists learn from radiometric dating?
A- how long ago a plant or animal lived
B- how much time it took a fossil to form
C- where a plant or animal lived
D- whether a fossil has modern relatives
Answers
Answer:
A- how long ago a plant or animal lived
starting with 0.050 mole cu2 and 0.50 mol nh3 in a 1.00 l container. calculate the [cu2 ] at equilibrium.
Answers
We can define a quantity called the equilibrium constant \(K_{c}\) based on the concentrations of all the different reaction species at equilibrium, which is also sometimes written as \(K_{eq}\) or K.
Because the equilibrium constant describes the molar concentrations, at equilibrium for a specific temperature, the c in the subscript stands for concentration \(\frac{mol}{L}\).
At equilibrium, the equilibrium constant can tell us whether the reaction has a higher concentration of products or reactants. We can also use \(K_{c}\) to see if the reaction has already reached equilibrium.
If any of the reactants or products are gases, the equilibrium constant can also be expressed in terms of the partial pressure of the gases. We typically use that value as \(K_{p}\) to distinguish it from the equilibrium constant when using molar concentrations \(K_{c}\).
To learn more about equilibrium constant, please refer:
https://brainly.com/question/12593147
#SPJ4
![starting with 0.050 mole cu2 and 0.50 mol nh3 in a 1.00 l container. calculate the [cu2 ] at equilibrium.](https://i5t5.c14.e2-1.dev/h-images-qa/answers/attachments/IMDVacmSWnpc39QQ5Q1kEIyaBKtjtUXQ.png)
City A in the Southern Hemisphere and City B in the Northern Hemisphere are located at the same latitude. Which statement is likely true about these
cities?
City B has the larger annual temperature range.
Both cities should have nearly identical winter temperatures.
City A has the larger annual temperature range.
Both cities likely have the same annual temperature range.
Answers
Answer:
City B has the larger annual temperature range
Explanation:
This is correct option because generally the northern side of the equator is high in temperature than the southern hemisphere part.
Since the southern side of the equator or Southern Hemisphere, where city A resides will generally have higher altitude or rise, so this creates higher average temperature.
How many grams of NaCI must be added to 2.00ml of water to make a 85.5m solution
Answers
The picture below shows a sample from a tree.
What kind of tree is this?
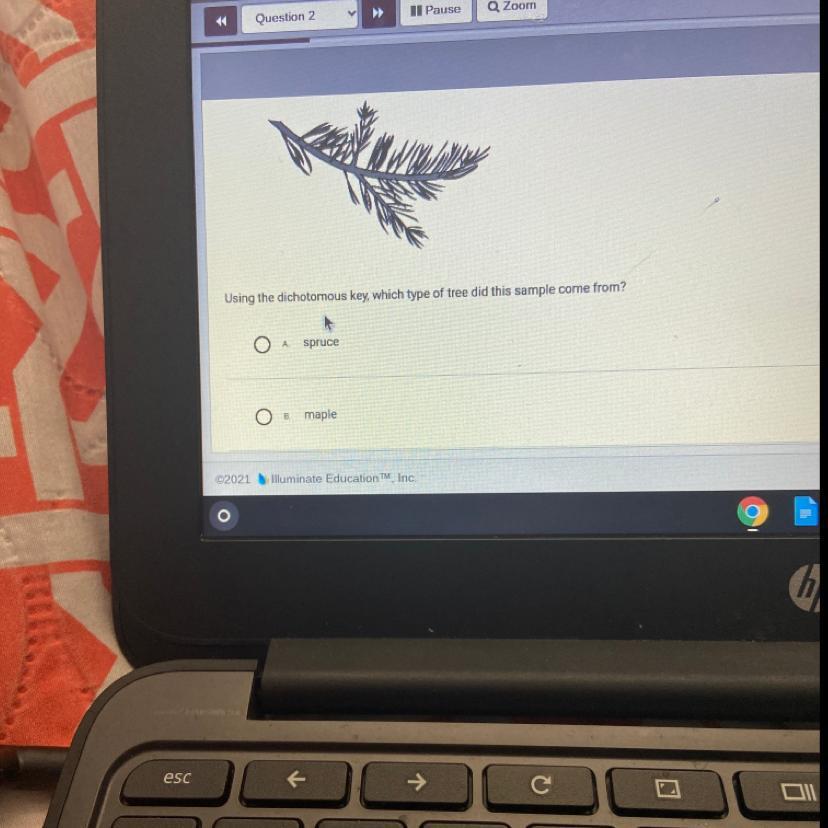
Answers
Answer:
I believe it is Spruce, because of the shape of the leaves
Answer:
Explanation:
Spruce
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
what is plutos element on the periodic table
Answers
Answer:
Discovery date 1940
Discovered by Glenn Seaborg and colleagues
Origin of the name Plutonium, is named after the then planet Pluto, following from the two previous elements uranium and neptunium.
Explanation:
Answer:
It's “Pu.”
Explanation:
“Pu” stands for plutonium, the element named for Pluto, back in 1941 it was the newest, teeniest planet in the solar system.
A firm chooses an order-up-to level of 80 units. At the start of this week there are no on-hand units, 20 on-order units, and 5 backordered units. How much should be ordered this week
Answers
The firm should order 65 units this week to meet the order-up-to level of 80 units and cover the backordered units.
To determine how many units a firm should order this week given an order-up-to level of 80 units, no on-hand units, 20 on-order units, and 5 backordered units, follow these steps:
1. Calculate the total units needed: Order-up-to level (80) minus on-hand units (0) equals 80 units needed.
2. Subtract the on-order units from the total units needed: 80 units needed minus 20 on-order units equals 60 units still required.
3. Account for the backordered units: 60 units still required plus 5 backordered units equals 65 units.
So, the firm should order 65 units this week to meet the order-up-to level of 80 units and cover the backordered units.
Learn more about backordered units. at https://brainly.com/question/29869731
#SPJ11
based on results presented in the passage, researchers hoping to alter the appearance of sgbp while maintaining its function as a cp providing a colored appearance would most logically choose to mutate which sgbp residue?
Answers
The nucleotide sequence of an organism's genome, that of a virus, extrachromosomal DNA, or other genetic components can change permanently in a process known as mutation.
Any alteration to a cell's DNA sequence. Mistakes in cell division can result in mutations, as can exposure to environmental DNA-damaging substances.
Gene mutations can be divided into two categories: small-scale mutations and large-scale mutations.
Appearance Alteration is the capacity to modify another person's skin, hair, and vocal chords (also known as adaptive appearance manifestation).
The genes that encode our pigment's sensitivity to color can multiply themselves throughout time. The additional copies are susceptible to mutations that change the range of wavelengths they can absorb.
To know about function https://brainly.com/question/11624077
#SPJ4
Which of the following is/are chemical changes? Select all that apply.
1. water boiling
2. vinegar and baking soda mixing and creating bubbles
3. dumping baking soda into water
4. a bike rusting while it sits outside
Answers
for fires to exist, four major elements must be present at the same time. they are hydrogen, fuel, heat for ignition, and the chemical reaction of fire.
Answers
For a fire to exist, four elements must be present simultaneously: hydrogen, fuel, heat for ignition, and the chemical reaction of fire. These elements are essential components of the fire triangle, which represents the conditions necessary for combustion to occur.
The combination of these elements leads to the sustained release of energy in the form of fire.
The fire triangle concept highlights the interdependence of the four elements required for a fire to exist. First, hydrogen refers to any substance that can act as a reducing agent, supplying electrons to support the combustion process. It is one of the components that contribute to the fuel source.
Fuel is the material that undergoes combustion and provides the necessary energy to sustain the fire. This can be solid, liquid, or gaseous substances that are capable of undergoing chemical reactions with oxygen.
Heat is the energy input required to initiate the ignition and sustain the chemical reactions involved in the fire. It raises the temperature of the fuel to its ignition point, at which combustion can occur.
The chemical reaction of fire involves the exothermic reaction between the fuel and oxygen, resulting in the release of heat and the production of combustion byproducts such as smoke, gases, and ash.
When all four elements are present simultaneously, a fire can ignite and propagate. Understanding and managing these elements are crucial for fire prevention, suppression, and safety measures.
Learn more about fire to exist from the given link:
https://brainly.com/question/33533266
#SPJ11
Describe the formation of calcium chloride (4 marks)
Answers
Answer:
It is an ionic compound that consists of one calcium cation Ca2+ and two chlorine anions. The bivalent calcium metal forms an ionic bond with two chlorine atoms. The formation of cacl2 happens mainly by reacting limestone (CaCo3) with hydrochloric acid (HCl).
Issue like gay marriage, abortion, and education have caused debate over the constitution because of the issue of what? (Hint: federalism)
Answers
44.) An object possesses a density of 2.780 g/mL. If the mass of the object is 0.896 grams, what is the
volume, in of the object in mL?
(A) 0.3223 mL
(B) 3.22 x 10-4 mL
(C) 3.223 x 10-4 mL
(D) 0.322 mL
Answers
An object possesses a density of 2.780 g/mL. and the mass of the object is 0.896 grams, the volume, in of the object in mL is (A) 0.3223 mL
To calculate the volume of an object, we can use the formula:
Volume = Mass / Density
Given that the mass of the object is 0.896 grams and the density is 2.780 g/mL, we can substitute these values into the formula:
Volume = 0.896 g / 2.780 g/mL
By performing the division, we find:
Volume = 0.3223 mL
So, the volume of the object is 0.3223 mL.
Therefore, the correct answer is (A) 0.3223 mL.
It's important to note that the volume is expressed in milliliters (mL) since the density is given in grams per milliliter (g/mL). The calculation involves dividing the mass (in grams) by the density (in g/mL), resulting in the volume in milliliters.
Understanding and applying the formula for calculating volume using mass and density helps us determine the physical space occupied by an object based on its characteristics. Therefore, Option A is correct.
Know more about Volume here:
https://brainly.com/question/14197390
#SPJ8
what is the reason of the gas pressure.
Answers
Answer:
The rapid motion and collisions of molecules with the walls of the container causes pressure (force on a unit area). Pressure is proportional to the number of molecular collisions and the force of the collisions in a particular area. The more collisions of gas molecules with the walls, the higher the pressure
The large number before a molecule in a chemical formula is called a _______________.
O subscript
O coefficient
O equation
Answers
Answer:
Subscript
Explanation:
The little number you see to the right of the symbol for an element is called a subscript. That number indicates the number of atoms of that element present in the compound. When balancing an equation, you can change the coefficients but not the subscripts.
hope this helps! if not, it's got to be coefficient or equation