the oxygen binding by hemocyanins is mediated by a) an iron ion b) a pair of iron ions c) a heme group d) a copper atom e) a pair of copper atoms
Answers
The oxygen binding by hemocyanins is mediated by d) a copper atom.
Hemocyanins are copper-containing proteins found in the blood of some invertebrates, such as mollusks and arthropods. The copper atoms in hemocyanins bind with oxygen molecules, allowing the transport of oxygen throughout the organism's body.
Unlike hemoglobin in vertebrates, which uses iron ions to bind with oxygen, hemocyanins use copper atoms. The copper atoms in hemocyanins form a complex with oxygen molecules, which gives the protein a blue color. This process is essential for the survival of many invertebrates that rely on hemocyanins for oxygen transport.
The oxygen binding by hemocyanins is mediated by e) a pair of copper atoms. Hemocyanins are respiratory proteins that use copper ions, rather than iron ions, for oxygen transport. These copper atoms work together to bind oxygen, allowing hemocyanins to carry out their oxygen transport function in invertebrates such as mollusks and arthropods.
Learn more about Hemocyanins
brainly.com/question/30811983
#SPJ11
Related Questions
the water in a beaker has a volume of 50 millimeters, is this an extensive property?
Answers
No, the volume of water in a beaker is not an extensive property.
Extensive properties are those that depend on the amount or size of the substance being measured. In other words, they are properties that change with the quantity of the substance. Examples of extensive properties include mass, volume, and total energy.
In the given scenario, the volume of water in the beaker is 50 milliliters. This volume remains the same regardless of the quantity of water present. Whether it's 50 milliliters or 500 milliliters, the volume measurement does not change. Therefore, the volume of water in the beaker is an example of an intensive property.
Intensive properties are independent of the amount or size of the substance. They are characteristics that remain constant regardless of the quantity of the substance. Examples of intensive properties include temperature, density, and color.
It's important to note that the distinction between extensive and intensive properties depends on the specific property being considered. While volume is typically an extensive property for a bulk substance, in the case of a fixed volume of water in a beaker, it becomes an intensive property.
In summary, the volume of water in a beaker is not an extensive property but rather an intensive property because it does not change with the quantity of the substance.
For more such questions on extensive property visit:
https://brainly.com/question/13055036
#SPJ8
The red and yellow diamond in a NFPA sign represents what?
Answers
The red diamond tells how flammable the chemical compound is or how easily it catches fire.
The yellow diamond tells about reactivity how quickly the compound reacts with other materials.
What is the wavelength of electromagnetic radiation with a frequency of 5.00 x 10^12 Hz? What kind of electromagnetic radiation is this?
Answers
Answer: 5.98 × 10⁻⁵ m (Infrared)
Explanation:
wavelength = speed of light ÷ frequency
wavelength = (2.99 × 10⁸ m/s) ÷ (5.00 x 10¹² /s)
= 5.98 × 10⁻⁵ m
Since radiation in the range, 10⁻³ to 7 × 10⁻⁷ is considered Infrared radiation, then the wave is an infrared wave.
It is estimated that the total amount of oxygen (O₂) contained in BIFs is equivalent to 6.6% of the oxygen present in the modern atmosphere. This is quite impressive given that the atmosphere during Archaean and early Proterozoic times was largely devoid of oxygen! Therefore, this reflects the photosynthetic efficiency of the early biosphere, coupled with its operation over long periods of time. Knowing that the mass of the modern atmosphere is 5.01×10¹⁸ kg, of which 21% is oxygen, what is the mass (in kilograms) of oxygen contained within BIFs?
_____ ×10¹⁶ kg of O₂ contained in BIF deposits
Knowing that the molecular mass of O₂ is 32 g/ mole (0.032 kg/ mole ), how many moles of O₂ are contained within BIFs?
____ ×10¹⁸ moles of O₂ contained in BIF deposits
Now, let us think about iron (Fe). The total mass of BIF's globally is estimated at 5.0×10¹⁷ kg, wherein iron accounts for approximately 35% by mass. The atomic mass of iron is 55.8 g/mole(0.0558 kg/mole). What is the total mass of iron in BIFs in kilograms and moles?
_____ ×10¹⁷ kg of Fe contained in BIF deposits
_____ ×10¹⁸ moles of Fe contained in BIF deposits
Finally, take the values you have computed in units of moles, and express them as the molar ratio of iron (Fe) to oxygen (O₂) of BIFs. You can do this by dividing both sides of the ratio by the larger number (Fe in this case).
FeO₂=1 _____
Your calculated ratio above should fall between the Fe: O₂ molar ratios of both Hematite (1:0.75) and Magnetite (1:0.67). Which molar ratio is your calculated value closest to (meaning which iron component, Hematite or Magnetite, is the more dominate in BIFs)?
Answers
The calculated molar ratio of iron to oxygen in BIFs is 1.452.
Comparing this ratio to the molar ratios of Hematite (1:0.75) and Magnetite (1:0.67), we can see that the calculated value of 1.452 is closest to the Hematite molar ratio of 1:0.75. Therefore, Hematite is the more dominant iron component in BIFs.
To calculate the mass of oxygen contained within BIFs, we'll use the given information:
Total mass of the modern atmosphere = 5.01×10¹⁸ kg
Percentage of oxygen in the modern atmosphere = 21%
Mass of oxygen contained within the modern atmosphere = (5.01×10¹⁸ kg) × (0.21) = 1.051×10¹⁸ kg
Percentage of oxygen contained in BIFs = 6.6% (given)
Mass of oxygen contained within BIFs = (6.6% of 1.051×10¹⁸ kg) = 6.6/100 × 1.051×10¹⁸ kg = 6.9166×10¹⁶ kg
Therefore, the mass of oxygen contained within BIFs is 6.9166 × 10¹⁶ kg.
To calculate the number of moles of oxygen contained within BIFs, we'll use the molecular mass of O₂:
Molecular mass of O₂ = 0.032 kg/mole
Number of moles of oxygen contained within BIFs = (Mass of oxygen in BIFs) / (Molecular mass of O₂)
= (6.9166×10¹⁶ kg) / (0.032 kg/mole) = 2.1614375 × 10¹⁸ moles
Therefore, the number of moles of oxygen contained within BIFs is 2.1614375 × 10¹⁸ moles.
Next, let's calculate the mass of iron in BIFs:
Total mass of BIFs = 5.0×10¹⁷ kg
Percentage of iron in BIFs = 35%
Mass of iron contained within BIFs = (35% of 5.0×10¹⁷ kg) = 35/100 × 5.0×10¹⁷ kg = 1.75×10¹⁷ kg
To calculate the number of moles of iron contained within BIFs, we'll use the atomic mass of iron:
Atomic mass of iron = 0.0558 kg/mole
Number of moles of iron contained within BIFs = (Mass of iron in BIFs) / (Atomic mass of iron)
= (1.75×10¹⁷ kg) / (0.0558 kg/mole) = 3.1367419 × 10¹⁸ moles
Therefore, the number of moles of iron contained within BIFs is 3.1367419 × 10¹⁸ moles.
Finally, let's calculate the molar ratio of iron to oxygen in BIFs:
Molar ratio of iron to oxygen = (Number of moles of iron) / (Number of moles of oxygen)
= (3.1367419 × 10¹⁸ moles) / (2.1614375 × 10¹⁸ moles)
≈ 1.452
To know more about modern atmosphere
https://brainly.com/question/2508257
#SPJ11
1. ___is the speed and direction of movement.
1 Acceleration
2Density
3Velocity
4Work
Answers
Answer:
VELOCITY
Explanation:
VELOCITY IS THE SPEED AND DIRECTION OF MOVEMENT
Explanation:
velocity
reason: The velocity of an object is the rate of change of its position with respect to a frame of reference, and is a function of time. Velocity is equivalent to a specification of an object's speed and direction of motion (e.g. 60 km/h to the north).
Why is the reaction of ethene with bromine called an addition reaction?.
Answers
Answer: an addition reaction is one where two molecules react together to produce one. in this case a bromine molecule reacts with ethene and one of the bromine atoms is added to ethene
Explanation:
hope this helps :)
What drives an electrolytic cell?
A. A spontaneous redox reaction
O B. Electrolytic solutions
O C. An external voltage source
D. A salt bridge filled with ions
Answers
Answer:
C. An external voltage source
Explanation:
An electrolytic cell converts electrical energy from an external voltage source into chemical energy that drives a reaction.
The external voltage source is usually a battery. This energy form generated from the battery helps to drive chemical reactions.
The process involves the decomposition of an ionic compound by means of current passed into the aqueous or molten form of the compound through conductors known as electrodes.
Answer:
C. external voltage source
Explanation:
Just took the quiz
Create your very own cosmic address for your home or school using the following
template.
Name
street
city
state
country
planet
planetary system
Galaxy
Galaxy Group
Galaxy supercluster
Answers
The cosmic address would be as given below:
Name: Micky Michael Street : 120 White Bridge Road NashvilleCity: NashvilleState: Tennessee Country: United state of AmericaPlanet: EarthPlanetary system: Solar systemGalaxy: Milky way galaxyGalaxy group: Local galaxyGalaxy supercluster: Virgo superclusterWhat is a galaxy?A galaxy is a huge collection of gas, dust, and billions of stars and their solar systems.
The Sun is a part of the Milky Way Galaxy.
The cosmic address indicating my address in the universe will be: Micky Michael , 120 White Bridge Road Nashville, Nashville, Tennessee , United state of America, Earth, Solar system, Milky way galaxy, Local galaxy, Virgo supercluster in that order.
In conclusion, a cosmic address is an address showing where one can be found in the universe.
Learn more about galaxies at:https://brainly.com/question/1587314
#SPJ1
what are the factors affecting gravity?
Answers
Gravity, as a fundamental force of nature, is influenced by several factors. The following are some of the key factors affecting gravity:
Mass: The most significant factor affecting gravity is the mass of the objects involved. According to Newton's law of universal gravitation, the gravitational force between two objects is directly proportional to the product of their masses. Greater mass leads to a stronger gravitational force.Distance: The distance between two objects also plays a crucial role in the strength of gravity. According to the inverse square law, the gravitational force decreases as the distance between objects increases. As objects move farther apart, the gravitational attraction between them weakens.Gravitational Constant: The gravitational constant, denoted by G, is a fundamental constant in physics that determines the strength of the gravitational force. It is a universal constant and does not change, affecting the overall magnitude of gravity.Shape and Distribution of Mass: The distribution of mass within an object can influence the gravitational field it generates. Objects with a more compact and concentrated mass distribution will have a stronger gravitational pull compared to those with a more spread-out mass distribution.External Influences: Gravity can be influenced by external factors such as nearby celestial bodies or the presence of other forces. For example, the gravitational interaction between the Earth and the Moon affects tides on Earth's surface.Need this asap!!!
Which type of substance gives off hydroxide ions when dissolved in water?
Acid
Base
Gas
Metal
Answers
Answer:
Acid
Explanation:
A student measures the volume of a small irregularly- shaped stone. Which apparatus must be used? a. a measuring cylinder containing water and a ruler only. b. a measuring cylinder containing water only. c. an empty measuring cylinder and a ruler only. d. a ruler only.
Answers
Answer:
a measuring cylinder containing water only.
Explanation:
If we want to measure the volume of an irregular object. We need a properly calibrated measuring cylinder filled with water of known volume.
The irregular object is now inserted into the measuring cylinder containing water and the change in the volume of water is noted. The volume of the irregular object is now obtained as;
New volume of water - initial volume of water.
Hence, only a measuring cylinder filled with water is required to measure the volume of a small irregularly- shaped stone.
The following irreversible reaction A-3R was studied in the PFR reactor. Reactant pure A (CAO=0.121 mol/lit)is fed with an inert gas (40%), and flow rate of 1 L/min (space velocity of 0.2 min-1). Product R was measured in the exit gas as 0.05 mol/sec. The rate is a second-order reaction. Calculate the specific rate constants.
Answers
The specific rate constant of the second-order irreversible reaction is 122.34 L/mol.s.
A second-order irreversible reaction A-3R was studied in a PFR reactor, where reactant pure A (CAO=0.121 mol/lit) is fed with an inert gas (40%), and flow rate of 1 L/min (space velocity of 0.2 min-1). Product R was measured in the exit gas as 0.05 mol/sec.
To calculate the specific rate constant, we use the following equation:0.05 mol/sec = -rA * V * (1-X). The negative sign is used to represent that reactants decrease with time. This equation represents the principle of conservation of mass.Here, V= volume of the PFR. X= degree of conversion. And -rA= the rate of disappearance of A= k.CA^2.To calculate the specific rate constant, k, we need to use a few equations. We know that -rA = k.CA^2.We can also calculate CA from the volumetric flow rate and inlet concentration, which is CAO. CA = (CAO*Q)/(Q+V)The volumetric flow rate, Q = V * Space velocity (SV) = 1 * 0.2 = 0.2 L/min.
Using this, we get,CA = (0.121*0.2)/(1+0.2) = 0.0202 mol/LNow, we can substitute these values in the equation of rate.0.05 = k * (0.0202)^2 * V * (1 - X)The volume of PFR is not given, so we cannot find the exact value of k. However, we can calculate the specific rate constant, which is independent of volume, and gives the rate of reaction per unit concentration of reactants per unit time.k = (-rA)/(CA^2) = 0.05/(0.0202)^2 = 122.34 L/mol.
Learn more about specific rate constant:
https://brainly.com/question/33346381
#SPJ11
Which of the following etiologic agents results in the formation of abscesses? A) Staphylococcus B) Mycoplasma C) Streptococcus D) Blastomyces
Answers
The etiologic agent that results in the formation of abscesses is Staphylococcus.
What are Etiologic agents?
Etiologic agents are the pathogens that can cause diseases, illnesses, or infections. It is a substance or organism that can cause or induce a disease. Etiologic agents can be bacteria, viruses, fungi, and parasites. The bacteria that causes infections, particularly abscesses, are called Staphylococcus. The bacterial infection that results in the formation of abscesses is medically referred to as staph infection.
Staphylococcus bacteria live on the skin and in the nose of about 1/3 of the population. Although, in most cases, the bacteria will not cause any problems. It is only when they enter the body that they can cause an infection. They can cause skin infections such as boils and impetigo, which can develop into abscesses. Hence, the etiologic agents that results in the formation of abscesses is Staphylococcus.
learn more about Staphylococcus on
https://brainly.com/question/26562205
#SPJ11
PLEASE HELP DUE AT 2PM I'M DESPERATE, WILL GIVE BRAINLEST!!
1. When magnesium is burned in air, its mass increases. Explain why this is.
2. When a match is burned in air, its mass decreases. Explain why this is.
3. This equation is unbalanced Na + F2 → NaF. Explain how you know it is unbalanced.
4. Why does it need to be balanced? Refer to the conservation of mass in your answer
5. A student heats up a metal and finds that its mass increases. The students says “this is because heat has been added.” Explain why this is incorrect.
Answers
Answer:
Ans no 1. As magnesium used to react with oxygen and due to the reaction the mass (weight) of magnesium increases , this reaction between oxygen and magnesium which form magnesium oxide in the air by forming white smoke increases the mass of magnesium
The atomic weight of iodine is less than the atomic weight of tellurium. However, Mendeleev listed iodine after tellurium in his original periodic table because he suspected the atomic weights for these elements were inaccurate. What was Mendeleev's reason for this suspicion?
Answers
Explanation :
As we know that Mendeleev arranged the elements in horizontal rows and vertical columns of a table in order of their increasing relative atomic weights.
He placed the elements with similar nature in the same group.
According to the question, the atomic weight of iodine is less than the atomic weight of tellurium. So according to this, iodine should be placed before tellurium in Mendeleev's tables. But Mendeleev placed iodine after tellurium in his original periodic table.
However, iodine has similar chemical properties to chlorine and bromine. So, in order to make iodine queue up with chlorine and bromine in his periodic table, Mendeleev exchanged the positions of iodine and tellurium.
As we know that the positions of iodine and tellurium were reversed in Mendeleev's table because iodine has one naturally occurring isotope that is iodine-127 and tellurium isotopes are tellurium-128 and tellurium-130.
Due to high relative abundance of tellurium isotopes gives tellurium the greater relative atomic mass.
What is the mass in grams of 2.3 moles of barium sulfate?
Answers
Answer:
536.79608
Explanation:
How many liters of oxygen are there in 5 grams of oxygen at STP?
Answers
Answer:
The volume of 5.0 g CO2 is 2.6 L CO2 at STP.
Explanation:
hope this helps :)
Which of the following is a solid fuel (multicorrect)
(a)Petrol
(b)Diesel
(c) Wax
(d)Coal
Answers
Answer:
the answer is coal
Explanation:
Help please help me!!!!!
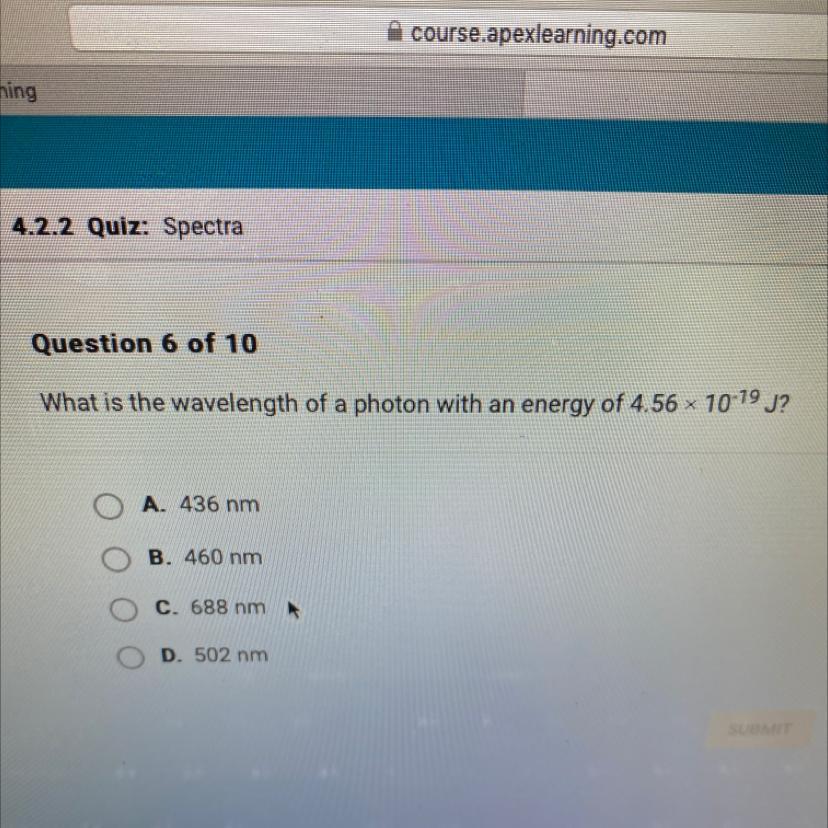
Answers
Answer:
B. 460 NM
Explanation:
THAT THE ANSWER
Nicotine is an alkaloid found in the nightshade family of plants (solanaceae) that acts as a stimulant in mammals. Nicotine is made up of 74% carbon, 8.7% hydrogen, and 17.3% nitrogen, what is the empirical formula?
What is the ratio of moles?
Answers
From the percentage composition, the empirical formula of nicotine is C₅H₇N.
The mole ratio of the elements carbon, hydrogen, and nitrogen is 5 : 7 : 1
What is the empirical formula of a compound?The empirical formula of a compound is the formula showing the elements combined in the simplest ratio.
The empirical formula of nicotine is calculated as follows:
Mole ratio of Carbon : 74%/12 = 0.06 moles
Mole ratio of Hydrogen : 8.7%/1 = 0.087 moles
Mole ratio of nitrogen : 17.3% / 14 = 0.012 moles
Simplest mole ratio:
Carbon: 0.06/0.012 = 5
Hydrogen: 0.087 / 0.012 = 7
Nitrogen : 0.012 / 0.012 = 1
The empirical formula of nicotine will be C₅H₇N
Learn more about empirical formula at: https://brainly.com/question/1603500
#SPJ1
How did ethanol use affect the shortage of corn available to consumers during and after the drought of 2012?.
Answers
Ethanol use was effect the shortage of corn available on those time because of corn is an ingredient to produce the ethanol. Hence, ethanol use was aligned with the rarity of corn availability on those time. the more ethanol was used, the more rare corn availability for consumer.
What is the effect of the drought?The drought is a condition where the area cant provide enough water supply for a long. Agriculture sector is the most affected sector when the drought occur. The drought cause crop failure, and this condition create domino effect on another sector such as food price get more expensive, and because of food get more expensive, buying power getting low cause small business out of business.
Learn more about drought here
https://brainly.com/question/12686086
#SPJ4
The presence of specific ________ signals that a given water source might be contaminated with pathogens.
Answers
The presence of specific bacteria, viruses, or other microorganisms that are known to be indicators of fecal contamination can signal that a given water source might be contaminated with pathogens.
These indicators include Escherichia coli (E. coli), coliform bacteria, enterococci, and fecal coliform bacteria. Testing for the presence of these indicators can help determine if the water source is safe for human consumption. It is important to note that the absence of these indicators does not necessarily mean that the water is free of pathogens, but their presence is a strong indication of contamination.
The presence of specific indicator organisms signals that a given water source might be contaminated with pathogens. Indicator organisms are microbes, such as coliform bacteria, that are commonly found in fecal matter and can signal the presence of more harmful pathogens. When these indicator organisms are detected in a water source, it suggests that there might be contamination, and further testing is needed to determine the presence and level of harmful pathogens.
Learn more about Escherichia coli
brainly.com/question/10581009
#SPJ11
You can use one-snip test to identify monophyletic groups − meaning that if you cut any branch on a tree, everything that falls off is a monophyletic group. why is this valid?
Answers
The one-snip test is a way to identify monophyletic groups in a phylogenetic tree, which is a diagram that shows the evolutionary relationships between species.
A monophyletic group is defined as a group of organisms that share a common ancestor and all of its descendants. When using the one-snip test, a branch is selected and cut, and everything that falls off is considered a monophyletic group. This test is valid because it ensures that all descendants of the cut branch share a common ancestor and are therefore considered a monophyletic group. If the test produces a monophyletic group, it means that the branching pattern in the tree correctly represents the evolutionary relationships between species, and that the group can be considered a clade, or a group of organisms with a shared ancestry.
Learn more about one-snip test:
brainly.com/question/12704015
#SPJ4
Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
A 0.250 l sample of 0.85 m hno3 is diluted to 750 ml. what is the molarity of the resulting solution?
Answers
The molarity of the resulting solution after diluting a 0.250 L sample of 0.85 M HNO3 to 750 mL is 0.283 M.
The molarity (M) of a solution is defined as the number of moles of solute per liter of solution. To calculate the molarity of the resulting solution, we can use the dilution formula: M1V1 = M2V2where M1 and V1 represent the initial molarity and volume of the concentrated solution, and M2 and V2 represent the final molarity and volume of the diluted solution. M1 = 0.85 M, V1 = 0.250 L, and V2 = 750 mL (which is equivalent to 0.750 L), we can rearrange the formula to solve for M2: Therefore, the molarity of the resulting solution is approximately 0.283 M.
M2 = (M1 * V1) / V2
M2 = (0.85 M * 0.250 L) / 0.750 L
M2 ≈ 0.283 M
Learn more about molarity here;
https://brainly.com/question/23306878
#SPJ11
What material is MOST effective in washing off explosive residue?
A. kerosene
B. gasoline
C. hydrocarbons
D. acetone
Answers
The material which is most effective in washing off explosive residue is referred to as acetone and is therefore denoted as option D.
What is an Explosive?This is referred to as a substance or device that can be made to produce a volume of rapidly expanding gas in an extremely brief period.
Explosive residue which is referred to as fragments of a container in which the residue corresponds to the shell of the container usually have most plastic type of stains that cannot be dissolved by other dryside agents. Acetone on the other hand is used to reduce or even remove many difficult stains such as paints, and the residues which is therefore the reason why it was chosen as the correct choice.
Read more about Explosive here https://brainly.com/question/16551803
#SPJ1
C
Unit Test
Unit Test Review Active
G
If a person has the values for an object's density and volume, what value can be calculated?
the object's size
the object's mass
the shape the object forms in a container
the amount of space the object takes up
Answers
If a person has the values for an object's density and volume, they can calculate the object's mass. Hence option B) is correct.
If a person has the values for an object's density and volume, they can calculate the object's mass. Density is defined as the mass per unit volume of an object. Mathematically, density is calculated by dividing the mass of an object by its volume. Rearranging the equation, we find that mass is equal to the product of density and volume. Therefore, if the density and volume of an object are known, multiplying them together will yield the object's mass. The other options mentioned in the question are not directly calculated using density and volume. The object's size is a broader term that encompasses various dimensions and may not be specifically derived from density and volume alone. The shape the object forms in a container and the amount of space the object takes up are influenced by both the object's mass and its dimensions, which are not solely determined by density and volume. Therefore option B) is correct.
For more question on density
https://brainly.com/question/26364788
#SPJ11
What is the coordination number of the metal atom in the [Fe(NCS)(H 2 O) 5 ] 2+ complex?
Answers
Answer:
6
Explanation:
The coordination number of the metal atom in the [Fe(NCS)(H2O)5]2+ complex is 6.
This means that there are six surrounding atoms or molecules (five water molecules and one NCS ligand) directly bonded to the central iron (Fe) atom in the complex.
Hope it helps!!
The coordination number of the metal atom in the [Fe(NCS)(H2O)5]2+ complex is six.
Coordination number is the total number of neighbors (atoms or ions) that a central atom has in a coordination compound or a complex. Coordination compounds are chemical species that have a central metal ion bonded to a set of molecules or ions, known as ligands.
These ligands are capable of donating a pair of electrons to the central metal ion and thus, form coordinate covalent bonds.Coordination number of metal ion in [Fe(NCS)(H2O)5]2+ complex:In the given coordination compound, Fe(II) is the central metal ion that is coordinated with five water molecules and one NCS– anion ligand.
Thus, the total number of coordinate covalent bonds formed between Fe(II) and its ligands is 6.Therefore, the coordination number of the metal atom in the [Fe(NCS)(H2O)5]2+ complex is six.
Learn more about Coordination number
brainly.com/question/27289242
#SPJ11
Using the following equation:
2NaOH + H2SO4 = 2H2O + NaSO4
How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid?
Answers
Taking into account the reaction stoichiometry, 355 grams of Na₂SO₄ will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid.
Reaction stoichiometryIn first place, the balanced reaction is:
H₂SO₄ + 2 NaOH → Na₂SO₄ + 2 H₂O
By reaction stoichiometry, the following amounts of moles of each compound participate in the reaction:
H₂SO₄: 1 moleNaOH: 2 moles Na₂SO₄: 1 moleH₂O: 2 molesThe molar mass of the compounds is:
H₂SO₄: 98 g/moleNaOH: 40 g/moleNa₂SO₄: 142 g/moleH₂O: 18 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
H₂SO₄: 1 mole× 98 g/mole= 98 gramsNaOH: 2 moles× 40 g/mole= 80 gramsNa₂SO₄: 1 mole× 142 g/mole= 142 gramsH₂O: 2 moles× 18 g/mole= 36 gramsMass of Na₂SO₄ formedIt can be applied the following rule of three: if by reaction stoichiometry 80 grams of NaOH form 142 grams of Na₂SO₄, 200 grams of NaOH form how much mass of Na₂SO₄?
mass of Na₂SO₄= (142 grams of Na₂SO₄×200 grams of NaOH) ÷80 grams of NaOH
mass of Na₂SO₄= 355 grams
Finally, 355 grams of Na₂SO₄ are formed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
discharge by the combination of Nitrogen and oxygen
Answers
Answer:
NO2
Explanation:
N = Nitrogen
O = Oxygen
Answer:
NO. 2.
is the answer
hopes this helps