URGENT PLEASEEEE
QUESTION 47 In the small intestine, chylomicrons are picked up by lacteals; simple sugars and amino acids directly enter the blood. O True False QUESTION 48 Cytosol is intracellular fluid; most extrac
Answers
47. True.In the small intestine, chylomicrons, which are large lipoprotein particles, are indeed picked up by lymphatic vessels called lacteals. Chylomicrons contain dietary fats (triglycerides) and are responsible for transporting these fats from the small intestine to the lymphatic system and eventually to the bloodstream.
On the other hand, simple sugars and amino acids, which result from the digestion of carbohydrates and proteins, respectively, are directly absorbed into the bloodstream through the small intestine's capillaries. They do not require the lymphatic system for transport and enter the blood directly.
48.True.Cytosol is indeed the intracellular fluid present within the cytoplasm of cells. It is a gel-like substance that surrounds the organelles and other cellular structures. Cytosol consists of water, ions, small molecules, and various proteins that are dissolved or suspended within it. It plays a crucial role in cellular metabolism, transport of molecules, and the overall functioning of the cell.
To know more about intracellular , visit;
https://brainly.com/question/14445684
#SPJ11
Related Questions
100 POINTS! I WILL MARK AS BRAINLIEST FOR FIRST CORRECT ANSWER!!!
When iodine monochloride, ICl, and chlorine gas react in a closed container, the following equilibrium is established:
ICl(l) + Cl₂(g) ⇆ ICl₃(s)
Iodine monochloride, is a brown liquid, whereas chlorine is a yellowish-green gas and ICl₃ is a yellow crystalline solid.
More chlorine is then added to the equilibrium mixture.
a) State what you would observe.
b) What happens to the rate of the forward reaction compared with the backward reaction?
c) What effect does this have on the yield of ICl₃?
d) What happend to the rate of the forward and backward reactions when equilibrium is reached again?
Answers
Answer:
I think it is C
Explanation:
We have that the reaction and a decrease in the concentration of the reactants will favor the reverse reaction that is the decomposition of the yellow iodine trichloride and the formation of green chlorine gas and brown iodine chloride
Given that some green chlorine gas, which is part of the reactant, will be removed, the reverse reaction will be favored and initial concentration of the yellow iodine trichloride and the green chlorine gas will be reduced while the proportional concentration of the brown iodine chloride will increase and the mixture will become more brown.
Answer:
d because you don't like math or any subject
What is the mass percent when 66 grams of NaCl is dissolved in 255 grams of water?
Answers
INFORMATION:
We have:
- 66 grams of NaCl
- 255 grams of water
If the NaCl is dissolved into the water, we must find the mass percent
STEP BY STEP EXPLANATION:
To find the mass percent we must must use the next formula
\(\text{ mass percent}=\frac{\text{ mass of solute}}{\text{ mass of solution}}\times100\)In our case,
- the solute is NaCl
- the solution would be the sum of masses from NaCl and water
Then,
- mass of solute = 66 g
- mass of solution = 66 g + 255 g = 321 g
Finally, replacing in the formula,
\(\text{ mass percent}=\frac{66g}{321g}\times100=20.5607\)ANSWER:
The mass percent when 66 grams of NaCl is dissolved in 255 grams of water is 20.60%
Alex is on a special diet and needs to watch her intake of carbohydrates. Her doctor told her to maintain a 1,500 calorie per day diet and to make sure that these come from no more than 20% carbohydrates. She is snacking on pretzels and the nutrition label on the package says that there are 23 grams of carbohydrates per serving, which is 8% of the recommended daily allowance.
How many calories should Alex get from carbohydrates and what percentage of her total recommended intake of carbohydrates in grams is this snack? (1 gram of carbohydrate provides 4 Calories.)
Alex should get 300 Calories from carbohydrates, which is about 75 grams. This snack is 30% of her total grams of carbohydrates per day.
Alex should get 300 Calories from carbohydrates, which is about 33 grams. This snack is almost 70% of her total grams of carbohydrates per day.
Alex should get 300 Calories from carbohydrates, which is about 60 grams. This snack is about 38% of her total grams of carbohydrates per day.
Alex should get 300 Calories from carbohydrates, which is about 75 grams. This snack is 8% of her total grams of carbohydrates per day.
Answers
Answer: Alex should get 300 Calories from carbohydrates, which is about 33 grams. This snack is almost 70% of her total grams of carbohydrates per day.
Answer:
B
Explanation:
I think
Hg2(NO3)2 + Na2CO3 = NaNO3 + HgCO3 balance this
Answers
Answer:
Hg(NO3)2 + Na2CO3 --> 2NaNO3 + HgCO3
a set of dilutions ranging in concentration from 1x10-2 m to 2x10-2 m are used to prepare a standard curve. transmittances of the dilutions range from 12% to 88%. could this plot be used to determine the concentration of a sample that had a concentration of 1.0x10-3 m?
Answers
The correct option is (B) No, the solution is too dilute and would have a %T over 90% where Beer's Law does not apply.
The solution is too dilute, it will have lower than desired concentration of the desired substance. To make solution more concentrated, more of the desired substance must be added to the solution. This increases the concentration of the desired substance, allowing it to be more effective in the intended application.
The amount of the desired substance must be adjusted according to the desired concentration of the solution. In addition, the amount of other substances in the solution must be considered to ensure the desired concentration is not too high or too low. Too high concentration could produce an undesired reaction, while too low a concentration would decrease the efficiency of the desired reaction.
Full question:
A set of dilutions ranging in concentration from 1x10-2 M to 2x10-2 M are used to prepare a standard curve. Transmittances of the dilutions range from 12% to 88%. Could this plot be used to determine the concentration of a sample that had a concentration of 1.0x10-3 M?
A) No, the solution is too dilute and would have a %T under 10% where Beer's Law does not apply.
B) No, the solution is too dilute and would have a %T over 90% where Beer's Law does not apply.
C) Yes, simply extend the standard curve.
D) No, the solution is too concentrated and would have a %T over 90% where Beer's Law does not apply.
To know more about Dilute solution:
brainly.com/question/16926361
#SPJ4
how many flourine atoms are in 410 g of UF6
Answers
3.6 ×10²⁴ atoms fluorine are in 410 g of UF\(_6\). Fluorine is an atomic number 9 chemical element with both the symbol F.
What is fluorine?Fluorine is an atomic number 9 chemical element with both the symbol F. This is the smallest halogen as well as occurs as a very poisonous, pale yellow diatomic vapor under normal circumstances.
It is exceptionally reactive being the most electronegative active catalyst, reacting with all other elements save the light inert.
mole = 410 / 352.02 =1.16mole
number of atom= 1.16× 6.022×10²³=6.98×10²³
number of atom of fluorine =6× 6.98×10²³= 3.6 ×10²⁴ atoms
Therefore, 3.6 ×10²⁴ atoms fluorine are in 410 g of UF\(_6\).
To learn more about fluorine, here:
https://brainly.com/question/10700214
#SPJ1
what is the solvent in an icy glass of lemonade?responseswaterwaterlemon juicelemon juicesugarsugarice
Answers
Answer: The solvent in an icy glass of lemonade is water.
The solvent in an icy glass of lemonade is water. Water is the most abundant liquid in the world, and is essential to life as we know it. In an icy glass of lemonade, the water serves to dissolve the other ingredients and carry their flavors and aromas.
The other ingredients in lemonade usually consist of lemon juice, sugar, and sometimes ice. The lemon juice provides the tartness, the sugar adds sweetness, and the ice provides a cooling sensation. Together, these ingredients create a refreshing summertime beverage.
Learn more about solvent here:
https://brainly.com/question/30452436#
#SPJ11
H2SO oxidation number
Answers
The oxidation number of S in H2SO is +0.
The oxidation number of O in H2SO is -2.
The oxidation number of H in H2SO is +1
according to the quantum mechanical model of the atom, an orbital represents a: multiple choice question. a) position where an electron probably is b) point where an electron cannot be. c) a position an electron must be d) a surface beyond which electrons cannot go
Answers
According to the quantum mechanical model of the atom, an orbital represents a position where an electron probably is. So, the correct option is (a).
An atomic orbital is a function that describes the position and wave-like activity of an electron in an atom in terms of both atomic theory and quantum mechanics. This function can be used to determine the likelihood of discovering any atom electron in any particular area surrounding the nucleus.
The physical area or space where the electron may be calculated to be present, as predicted by the specific mathematical shape of the orbital, is sometimes referred to as an atomic orbital. Simple names for orbitals with angular momentum quantum numbers l = 0, 1, 2, and 3 are the s orbital, p orbital, d orbital, and f orbital.
Learn more about quantum mechanical model of the atom here:
https://brainly.com/question/3453444
#SPJ4
given the data below, what will be the standard cell potential for a cr/fe voltaic cell? cr3 (aq) 3e– → cr(s); eo = –0.74 v fe2 (aq) 2e– → fe(s); eo = –0.44 v
Answers
To find the standard cell potential of a Cr/Fe voltaic cell, you subtract the standard reduction potential of Fe from the standard reduction potential of Cr.
The given reduction half-reactions are:
Cr³⁺(aq) + 3e⁻ → Cr(s) E° = -0.74 V
Fe²⁺(aq) + 2e⁻ → Fe(s) E° = -0.44 V
Determine the standard cell potential?In a voltaic cell, the reduction half-reaction with the more positive reduction potential occurs at the cathode (site of reduction), while the half-reaction with the less positive reduction potential occurs at the anode (site of oxidation).
To determine the standard cell potential, we subtract the reduction potential of the anode (Fe) from the reduction potential of the cathode (Cr):
E°cell = E°cathode - E°anode
= (-0.74 V) - (-0.44 V)
= -0.30 V
Therefore, the standard cell potential for the Cr/Fe voltaic cell is -0.30 V.
The negative sign indicates that the cell reaction is spontaneous in the reverse direction, from Fe to Cr.
To know more about cell potential, refer here:
https://brainly.com/question/31961504#
#SPJ4
Plz help! I’m on a timer!

Answers
Answer: c. ceabd
Explanation: Substances that are more dense move to the bottom of the beaker. Substances that are less dense float to the top.
Question is in the image
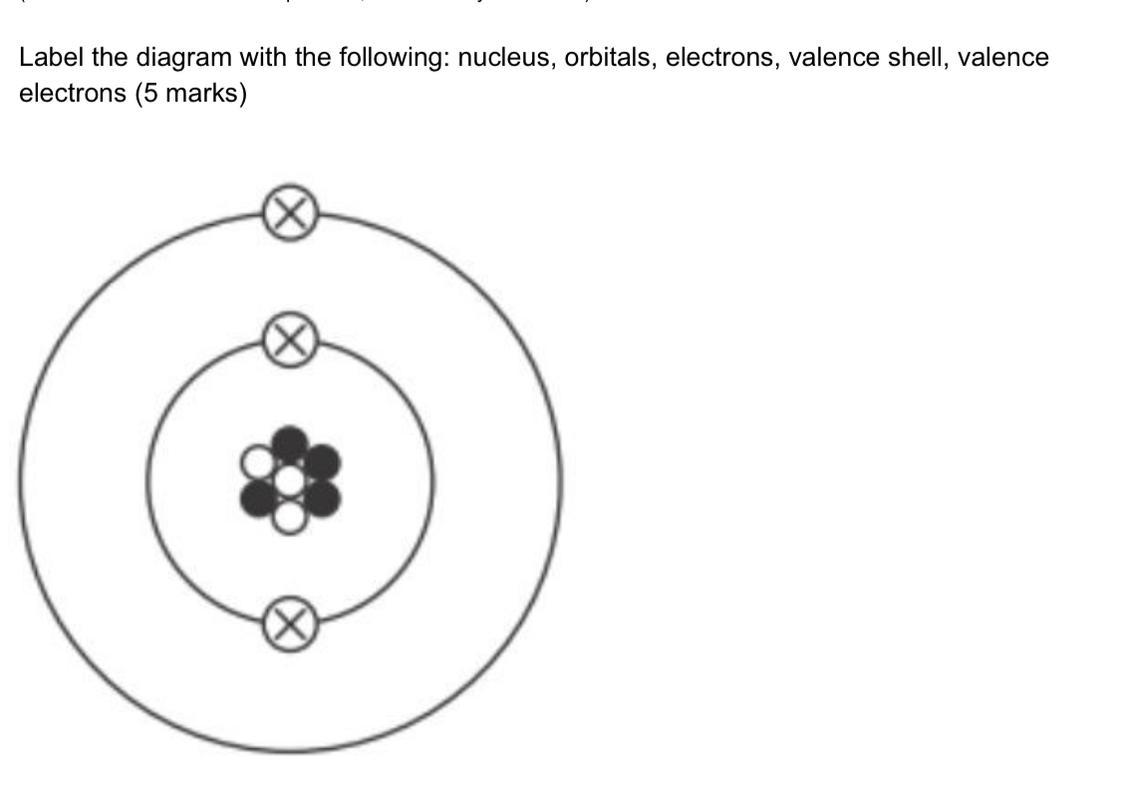
Answers
To see the number of atoms of an element in a given molecule we need to multiply stoichiometry to the number that is written on the foot of the element that is stoichiometry. Therefore, the given figure represents the atom.
What is atom?Atom is the smallest particle of any element, molecule or compound. Atom can not be further divided. Atoms contains nucleus in its center and electron that revolve around the atom in fixed orbit.
In the nucleus, proton and neutron are present. Electron has -1 charge while proton has +1 charge. Neutron is neutral that is it has no charge. So overall the charge of nucleus is due to only proton, not by neutron.
The circles that are in center represents nucleus. The two bigger circles represents orbitals. The cross sign represents electrons. The outermost bigger circle represents the valence shell and the electron in this shell represents the valence electron.
Therefore, the given figure represents the atom.
To know more about atom, here:
https://brainly.com/question/13518322
#SPJ1
1. B How many grams are in 5.00 mol of CO2? CO2 molar mass is 44 g/mol. 1 point Grams = Moles X Molar Mass
Answers
Answer: 220 g
Explanation:
To find the answer to this problem, all you need to do is multiply the number of moles by the molar mass of the molecule.
In this problem, you multiply 44 (the molar mass of CO2) by 5 (the grams of CO2 given) to get 220.
Answer:
220 g
Explanation:
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles CO2, or 44.0095 grams.
the henry's law constant for h2 is 8.1×10−4 matm at 25∘c. what pressure of hydrogen is needed to maintain a h2 concentration of 0.42 m?\
Answers
518.5 atm pressure of hydrogen is needed to maintain a \(H_2\) concentration of 0.42 m
The given Henry's law constant for \(H_2\) is 8.1 × 10^-4 M atm^-1 at 25°C. To find the pressure of hydrogen needed to maintain a \(H_2\) concentration of 0.42 M, we can use Henry's law.
The equation for Henry's law is:
C = kH*P
where C is the concentration of gas in moles per liter, P is the partial pressure of the gas in atmospheres, and kH is Henry's law constant in M/atm.
Plugging the values in Henry's law equation, we get:
0.42 = (8.1 × 10^-4)P
Dividing both sides by (8.1 × 10^-4), we get:
P = (0.42)/(8.1 × 10^-4)
P = 518.5 atm
Hence, the pressure of hydrogen needed to maintain a \(H_2\) concentration of 0.42 M is 518.5 atm.
Learn more about Henery's law here:
https://brainly.com/question/14908970
#SPJ11
the little circle subscripts at the top of the deltag, deltah,and deltas represent standard conditions . these conditions correspond to
Answers
The little circle subscripts at the top of the deltag, deltah, and deltas represent standard conditions. These conditions correspond to the standard atmospheric pressure, temperature, and humidity respectively.
The standard atmospheric pressure is the average atmospheric pressure at mean sea level, which is 1.01325 bar. The standard temperature is 20°C (68°F), and the standard humidity is 0.00% relative humidity.
Atmospheric pressure is measured in bar and is the amount of force per unit area exerted by the atmosphere on a surface. It is affected by factors such as the weather and altitude. Temperature is a measure of the kinetic energy of the particles in a substance and is measured in degrees Celsius (°C). Humidity is the amount of moisture in the air and is measured in relative humidity (%), which is the ratio of the partial pressure of water vapor in the air to the saturated vapor pressure at a given temperature.
In chemistry and thermodynamics, the values of deltag, deltah, and deltas are often used to calculate the enthalpy, Gibbs free energy, and entropy changes associated with a chemical reaction. The standard conditions for these subscripts are the most common values used when calculating the thermodynamic properties of a reaction. Knowing the standard conditions is important for predicting the thermodynamic behavior of a system.
To know more about enthalpy click on below link :
https://brainly.com/question/13996238#
#SPJ11
The empirical formula for a compound that contains 89.3% potassium and 10.7% nitrogen is:A)K2NB)KNC)K3ND)KN3
Answers
Explanations:
1. Calculate number of moles for K and N(i) Moles for K
mass = 89.3% = 89.3g
Mol. mass K =39.0983g/mol
∴ n = mass/molmass
=89.3/39.0988
=2.28 moles
(ii) Moles for N
mass = 10.7% = 10.7 g
Mol. mass N =14.0067g/mol
∴ n = mass/molmass
=10.7g/ 14.0067g/mol
=0.76 moles
2. Divide all components by the smallest value:• K= 2.28 /0.76 = 2.9898240669737 ≈ 3
• N=0.76/0.76 = 1
Therefore emperical formula has 3 K and 1 N = K3N4) How much of the world population lives in countries where there isn't enough water, or
the quality has been compromised?
Answers
Answer:
The answer is 1/3 of the population.
classify each species as a lewis acid or a lewis base. you are currently in a sorting module. turn off browse mode or quick nav, tab to items, space or enter to pick up, tab to move, space or enter to drop. lewis acid lewis base
Answers
To classify each species as a Lewis acid or a Lewis base, we need to understand the definitions of these terms.
A Lewis acid is a species that can accept an electron pair, while a Lewis base is a species that can donate an electron pair. Now, let's use this information for the sorting process.
Step 1: Identify the species you want to classify. (You have not provided any specific species, so I will provide a general guideline)
Step 2: Determine if the species can accept an electron pair (Lewis acid) or donate an electron pair (Lewis base). This is usually based on their electron configuration and the presence of vacant or lone electron pairs.
Step 3: Once you've determined whether the species is a Lewis acid or a Lewis base, you can sort them accordingly.
To know more about the Lewis acid and base refer here :
https://brainly.com/question/15570523#
#SPJ11
In a catalyzed reaction a reactant is often called a:.
Answers
Answer:
it is called a substrate
Explanation:
hopfully this helps u
Answer:
The reactant in an enzyme-catalyzed reaction is called a substrate
Stephan claims that Earth has both a gravitational field and a magnetic field. Which statement best supports his claim?
A.A compass points in the direction of both the magnetic and gravitational fields.
B.Any object will fall to the ground.
C.A bar magnet will fall to the ground.
D.Any object will fall to the ground, and a compass can be used for navigation.
Answers
Answer:
D
Explanation:
Without gravity, any object wont go to the ground, and without a magnetic field, a compass cant be used for navigation.
which of the following characteristics identifies a ph-balanced shampoo
Answers
The pH scale ranges from 0 to 14, with values below 7 considered acidic, 7 being neutral, and values above 7 being alkaline. Hair and scalp have a slightly acidic pH, and using a pH-balanced shampoo helps maintain the natural balance.
The characteristic that identifies a pH-balanced shampoo is having a pH level close to the natural pH level of the hair and scalp, which is around 4.5 to 5.5. Therefore, a pH-balanced shampoo will have a pH level in the acidic to neutral range, typically between 4.5 and 5.5, to avoid causing damage or disrupting the natural pH balance of the hair and scalp.
Learn more about natural pH here ;
https://brainly.com/question/5171852
#SPJ11
A pH-balanced shampoo should have a pH between 4.5 and 5.5, contain mild acids or bases, and help to keep the hair and scalp's natural pH level balanced.
Explanation:Characteristics of a pH-balanced shampoo:pH is between 4.5 and 5.5Contains mild acids or bases to maintain the desired pH level Helps to keep the hair and scalp's natural pH level balancedA pH-balanced shampoo is important because it prevents the scalp from becoming too dry or too oily. It ensures that the hair cuticle is closed, reducing frizz and improving shine. Using a pH-balanced shampoo can also help maintain the effectiveness of other hair products.
Learn more about pH-balanced shampoo here:https://brainly.com/question/32512053
#SPJ6
The paths that planets follow around the sun are called ____ , and they are shaped like an ellipse.
Answers
The paths that planets follow around the sun are called as orbit, and they are shaped like an ellipse.
What is orbit ?The orbits of the planets around the sun are elliptical. Kepler, an astronomer, provided a clear definition of these orbits. The three rules he created are referred to as Kepler's laws of planetary motion. It says that every planet does an orbit around the sun.
The plane of Earth's orbit around the sun serves as the definition of the ecliptic. The primary planets of our solar system, their moons, some asteroids, and their orbits all roughly follow this plane.
An orbit is a curved path taken by an object, such as a planet's path around a star, a natural satellite's path around a planet, or a man-made satellite's path around a planet, moon, or asteroid.
Thus, the paths that planets follow around the sun are called as orbit.
To learn more about an orbit, follow the link;
https://brainly.com/question/20435529
#SPJ12
find the pressure of 10.0 g of argon gas in 750 ml container at a temperature of 50 oc?
Answers
The pressure of 10.0 g of Ar gas in a 750 mL container at a temperature of 50°C is 5.56 atm.
The ideal gas law is given as:
PV = nRT
whereP is the pressure of the gas
V is the volume of the gas
n is the number of moles of the gas
R is the gas constant
T is the temperature of the gas.
Here, we are given the mass of the gas, the volume of the container and the temperature of the gas. We can use this information to determine the pressure of the gas.
Using the Ideal gas law
PV = nRT
Rearranging, we get:
P = nRT / VA
t standard temperature and pressure (STP),
1 mole of gas occupies 22.4 L and has a mass of 44 g (approx.)
The molar mass of Ar is 40 g/mol.
Using the above information, we can determine the number of moles of Ar present in the container.10.0 g Ar / 40 g/mol = 0.25 moles Ar750 ml = 0.75 LAr
Using the ideal gas law,
P = nRT / VP = 0.25 moles Ar x 0.082 L atm/mol K x (50 + 273.15) K / 0.75 LP = 5.56 atm
Therefore, the pressure of 10.0 g of Ar gas in a 750 mL container at a temperature of 50°C is 5.56 atm.
Learn more about ideal gas law here:
https://brainly.com/question/30458409
#SPJ11
8. Calculate the pH of a solution if the [OH-] concentration is 0.015 M.
A) 1.82
B) 8.82
C) 12.18
D) 4.32
Answers
Answer:
1.82
Explanation:
pH is given by the equation
Can someone please help me with this, ill give you a heart, a five star rating, and the brainliest answer!

Answers
Answer:
A not
B yes
C yes
D yes
Explanation:
A 2C 6H but 10O and 7O so no
B 3C 6O 2Fe on each side so yes
C 4H 1S 6O 2Na so yes
D 2Ag 2Cl 1Ca 2N 6O so yes
Giving brainliest for correct answer!

Answers
Question 7 options:
Birds are characterized by the presence of
, the defining characteristic of the group.
Answers
Answer:
True
Explanation:
Birds are characterized by the presence of certain physical features that are unique to the group and serve to distinguish birds from other animal groups. These features, such as feathers, a beak instead of teeth, a lightweight skeleton, and the ability to fly, are considered the defining characteristics of birds. These defining characteristics serve as the basis for classifying birds as a distinct group and are used by scientists to identify and study different bird species.
what two subatomic particles have to be different in order to have an ion?
A)Protons and electrons
B)Protons and neutrons
C)Neutrons and electrons
Answers
Answer:
A)Protons and electrons
Explanation:
An atom consist of electron, protons and neutrons. Protons and neutrons are present with in nucleus while the electrons are present out side the nucleus.
All these three subatomic particles construct an atom. A neutral atom have equal number of proton and electron. In other words we can say that negative and positive charges are equal in magnitude and cancel the each other. For example if neutral atom has 6 protons than it must have 6 electrons. The sum of neutrons and protons is the mass number of an atom while the number of protons are number of electrons is the atomic number of an atom.
when an atom lose or gain the electron ions are formed and number of protons and electrons no longer remain the same.
For example:
An atom of sodium when lose one electron it form positive ion. The number of electrons became 10 and number of protons remain 11.
Na → Na⁺ + 1e⁻
How much energy is released when 22.4g of CH4 is burned?
Answers
The combustion of methane, CH4, releases 890.4 kJ/mol. That is, when one mole of methane is burned, 890.4 kJ are given off to the surroundings. This means that the products have 890.4 kJ less than the reactants.
Combustion of one mole or 16 g of methane gas releases 810 kJ of heat energy. Thus, heat released by the combustion of 22.4 g of methane is 1134 kJ.
What is combustion?Combustion of a substance is its reaction with atmospheric oxygen producing carbon dioxide and water. Combustion of hydrocarbons gases takes places easily and can be burned easily for fuels.
The chemical equation of combustion of methane is written below:
\(\rm CH_{4} + 2O_{2} \rightarrow CO_{2} + 2H_{2}O\)
This is an exothermic process and heat is released from the reaction system.
The heat released by the combustion of one mole of methane is 810 kJ.
molar mass of methane = 16 g/mol
no.of moles in 22.4 g = 22.4/16 = 1.4 g
Thus, heat released by 1.4 moles = 1.4 ×810 kJ =1134 kJ.
To find more on combustion, refer here:
https://brainly.com/question/15117038?referrer=searchResult
#SPJ2
Which statement provides the best example of a scientific conclusion?
O A. Graphite can be used to make pencils.
O B. Diamond is mined in South Africa
O C. Diamond and graphite are both composed of carbon.
D. Diamond jewelry is prettier than graphite jewelry.
Answers
Answer:
c
Explanation:
In science, there is always processing of an item or sample to get an end product. So if the diamond is mined; there is a processing stage used to come out with the final product as Diamond through carbon in the heating process