What are some downsides to fossil fuels? (Select all that apply.)
Answers
Answer:
you did not show me the options so i'm just going to say a few.
Explanation:
global warming
releases carbon dioxide into the air
other harmful chemicals
takes up a lot of space and time
Related Questions
Match the name of each gas law to the properties it compares. Boyle's law Charles's lawGay-Lussac's lawA) Pressure and volume B) Temperature and volume C) Pressure and temperature
Answers
Answer:
Boyle's law: A) Pressure and volume.
Charles's law: B) Temperature and volume.
Gay-Lussac's law: C) Pressure and temperature.
Explanation:
Hello,
In this case, since Boyle's law study the relationship between pressure and volume at constant temperature as an inversely proportional one, we have:
Boyle's law: A) Pressure and volume.
Next, since the Charles' law study the relationship between the volume and the temperature at constant pressure as a directly proportional one, we have:
Charles's law: B) Temperature and volume.
Then, since the Gay-Lussac's law study the relationship between the pressure and the temperature at constant volume as a directly proportional one as well, we have:
Gay-Lussac's law: C) Pressure and temperature.
Best regards.
Answer:
D, B, E
Explanation:
The natural abundance of 13C is 1.1%. What are the relative peak heights of the M and M 1 peaks in the mass spectrum of decane
Answers
The relative peak height of the M+1 peak is 0.104, and the relative peak height of the M peak is 0.896.
The relative peak heights of the M and M+1 peaks in the mass spectrum of decane are determined by the percentage of carbon-13 isotopes present in the sample.
The M peak represents the molecular ion, which is the parent molecule after losing an electron. It has a mass-to-charge ratio (m/z) equal to the molecular weight of the compound. The M+1 peak represents the molecular ion that contains one carbon-13 atom. It has a mass-to-charge ratio that is one unit higher than the M peak.
Decane has a molecular formula of C₁₀H₂₂, which means it contains 10 carbon atoms. If the natural abundance of 13C is 1.1%, then the probability of finding a carbon atom that is 13C is 0.011.
The probability of finding 10 carbon atoms that are all 12C is (0.989)10, which is approximately 0.904. The probability of finding 9 carbon atoms that are 12C and 1 carbon atom that is 13C is 10(0.989)9(0.011), which is approximately 0.094.
This means that the M peak is about 8.6 times higher than the M+1 peak in the mass spectrum of decane.
To know more about natural abundance click on below link:
https://brainly.com/question/2496431#
#SPJ11
In this lab you will construct several electrochemical cells where both half-cells contain a copper electrode in a copper (II) solution. What standard cell potential (Eºcell would be expected for a voltaic cell comprised only of copper? O 0.68 V O 0.34 V 0 0.0592 V O 0.00 V 0 -0.34 V
Answers
In the lab, electrochemical cells are being constructed with copper electrodes in copper (II) solution for both half-cells. The expected standard cell potential (Eºcell) for a voltaic cell consisting solely of copper is 0.00 V.
The standard cell potential, denoted as Eºcell, represents the potential difference between the two half-cells of an electrochemical cell. It is a measure of the cell's ability to generate an electric current. In this case, since both half-cells contain copper electrodes in copper (II) solution, there is no difference in the reduction potential of the two half-reactions. This results in an equal and opposite potential, leading to a net cell potential of zero. This implies that no spontaneous redox reaction occurs and no electrical energy is generated or consumed within the cell.
For more information on electrochemical potential visit: brainly.com/question/31868529
#SPJ11
When 25 g of a metal at 91 ◦C is added to
53 g of water at 28 ◦C, the temperature of the
water rises to 36.5
◦C. What is the specific
heat capacity of the metal? Assume no heat
was lost to the surroundings.
Answer in units of J
g ·
◦C
.
Answers
The specific heat capacity of the metal is 0.094 J/g·°C.
The specific heat capacity of the metal can be calculated using the equation Q = mcΔT,
First, calculate the heat absorbed by the water:
Q = (53 g) x (4.18 J/g·°C) x (36.5 °C - 28 °C) = 1,531.6 J
Since no heat is lost to the surroundings, the heat lost by the metal is equal to the heat absorbed by the water.
Q = (25 g) x (c metal) x (36.5 °C - 91 °C) = -16250 c metal
Solving for c metal yields:
c metal = (1,531.6 J) / (16250 J) = 0.094 J/g·°C
To know more about specific heat capacity, here
brainly.com/question/29766819
#SPJ1
In one of NASA's space tether experiments, a 20.0 km-iong conducting wire was deployed by the space shuttle as it orbited at 7.86×10^3m/s around Earth and across Earth's magnetic field lines. The resulting motional emf was used as a power source. If the component of Earth's magnetic field perpendicular to the tether was 1.31×10^−5T, determine the maximum possible potential difference (in V) between the two ends of the tether. 2,375 V 1,900 V 1,980 V 2,130 V 2,060 V 1,840 V 2,120 V
Answers
The maximum possible potential difference between the two ends of the tether is approximately 2.06 × 10³ V. Thus, the correct answer is 2.06 × 10³ V.
The maximum possible potential difference (V) between the two ends of the tether can be calculated using the formula:
V = B * L * v
where B is the magnetic field strength, L is the length of the wire, and v is the velocity of the wire.
In this case, we have the following values:
B = 1.31 × 10⁻⁵ T (magnetic field strength)
L = 20.0 km = 20,000 m (length of the wire)
v = 7.86 × 10³ m/s (velocity of the wire)
Plugging these values into the formula, we can calculate the potential difference:
V = (1.31 × 10⁻⁵ T) * (20,000 m) * (7.86 × 10³ m/s)
Calculating this value:
V ≈ 2.06 × 10³ V
Therefore, the maximum possible potential difference between the two ends of the tether is approximately 2.06 × 10³ V. Thus, the correct answer is 2.06 × 10³ V.
Learn more about potential difference from the link given below.
https://brainly.com/question/23716417
#SPJ4
Structure of 2,4,5-trimethyl-4,(1-methylethyl)heptane
Answers
The structure of 2,4,5-trimethyl-4,(1-methylethyl)heptane is a long-chain hydrocarbon with seven carbon atoms. It contains three methyl (CH3) groups attached to carbons 2, 4, and 5, and an isopropyl (C3H7) group attached to carbon 4. The prefix "trimethyl" indicates the presence of three methyl groups, while "4,(1-methylethyl)" indicates the location of the isopropyl group on carbon 4.
The name "2,4,5-trimethyl-4,(1-methylethyl)heptane" provides important information about the structure of the compound. Starting with the parent hydrocarbon, heptane, which consists of seven carbon atoms, the prefix "trimethyl" indicates that there are three methyl (CH3) groups attached to the carbon backbone. These methyl groups are located on carbons 2, 4, and 5 of the heptane chain.
Additionally, the term "4,(1-methylethyl)" specifies the presence of an isopropyl (C3H7) group attached to carbon 4. The "4" indicates the position of the isopropyl group on the carbon chain, while "(1-methylethyl)" represents the chemical structure of the isopropyl group, which consists of a methyl (CH3) group attached to a secondary carbon (C) atom.
Combining all the information, the structure of 2,4,5-trimethyl-4,(1-methylethyl)heptane can be visualized as a long-chain hydrocarbon with seven carbon atoms, three methyl groups on carbons 2, 4, and 5, and an isopropyl group attached to carbon 4.
To learn more about Hydrocarbon - brainly.com/question/30666184
#SPJ11
What is the mass percent of nitrogen in NH4Cl?
Answers
Answer:
Explanation:I believe the mass percent of nitrogen in NH4Cl N H 4 C l is 26.19%. I might be right but I had this same question on my eoc
Answer:
So, 53.49 g (M) of NH4Cl N H 4 C l contains 14.01 g (m) of N . So, the mass percent of nitrogen in NH4Cl N H 4 C l is 26.19%.
Explanation:
the answer is 26.19%
WHAT IS NH4CH
It was reborn in the united states people used it for equants
Learn more: brainly.com/question/20838718
Potassium bromide conducts electricity when ______
Answers
Answer:
When melted or dissolved in water.
Explanation:
Potassium bromide in its solid form contains ions, which are charged atoms. Through the heating process, the melted potassium bromide becomes an ionic liquid. If solid potassium bromide is dissolved, for example in water, the resulting release of ions allows it to conduct electricity.
Complete la siguiente comparación de similitud:
Molécula ES A enlace covalente; como: __________________ ES A enlace iónico.
Iones
Metaloides
Red cristalina
Answers
Answer:
metaloides
Explanation:
Arrange these gases in order of solubility, NH3, N2 CO2
Answers
The correct increasing order of solubility for the given gases is: NH₃ < N₂ < CO₂
What does solubility mean?A chemical's solubility is the maximum amount of that substance that will dissolve in a specific amount of solvent at a specific temperature. Solubility has several practical applications, including water filtering, beverage manufacturing, and vitamin storage.
Why is solubility important?The ability of a medicine to dissolve is essential to its effectiveness. A drug substance could be absorbed without it, which results in limited bioavailability. Drugs with poor solubility can potentially cause problems with metabolism or permeability, interaction with other medications, or the requirement for prolonged drug release.
To know more about Solubility visit:
https://brainly.com/question/8591226
#SPJ1
Calculate the answers to the appropriate number of significant numbers
32.567
135.0
+ 1.4567
—————
Answers
Answer:
32.567+135.0+1.4567=169.027
to significant figures is 16
Can anyone help? Thank you!

Answers
Answer:
Six oxygen atoms are involved in this reaction.
How much energy is required to raise the temperature of 3 kg of lead from 15°C to 20°C? Use the table below and this equation: Q = MCAT.
The question is written right above the table given.
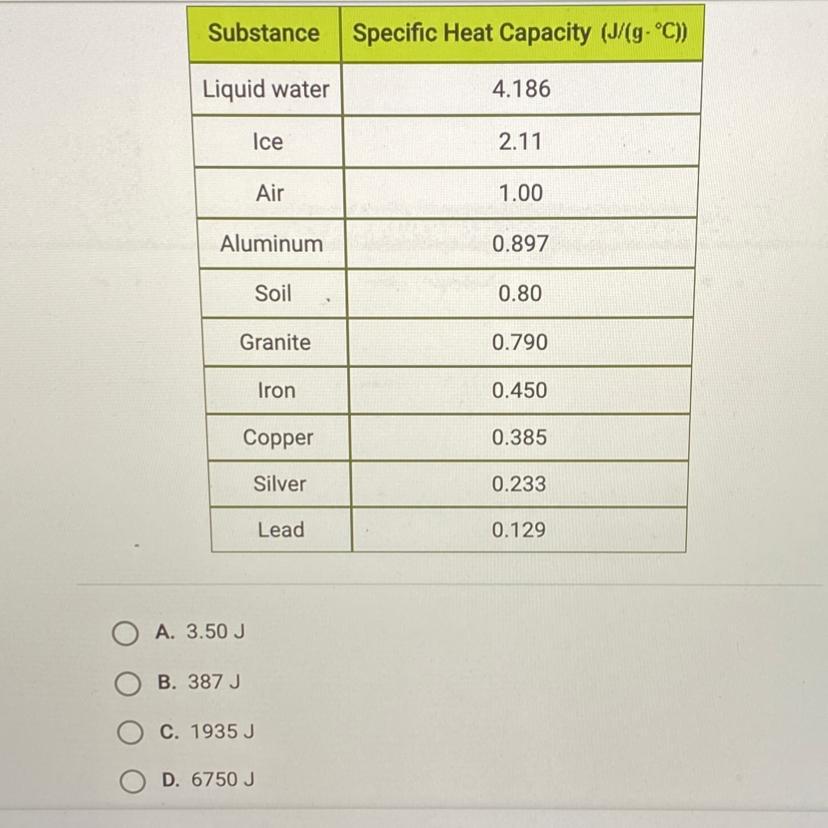
Answers
Answer:
1935J
Explanation:

Answer:
\(\boxed {\boxed {\sf C. \ 1935 \ J}}\)
Explanation:
The equation for this problem is:
\(q=mc\Delta T\)
where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
The mass is 3 kilograms, but the specific heat capacity includes grams in the units. Convert kilograms to grams. There are 1000 grams in 1 kilogram.
\(\frac {1000 \ g}{1 \ kg}\)\(3 \ kg *\frac {1000 \ g}{1 \ kg}\)\(3 *1000 \ g = 3000 \ g\)The specific heat capacity for lead is found on the table. It is 0.129 J/g°C.
Let's find the change in temperature. It is raised from 15 °C to 20 °C.
\(\Delta T= final \ temperature - initial \ temperature \\\Delta T= 20 \textdegree C - 15 \textdegree C\\\Delta T= 5 \textdegree C\)Now we know every value.
m= 3000 g c= 0.129 J/g°CΔT= 5 °CSubstitute the values into the formula.
\(q= (3000 \ g)( 0.129 \ J/g \textdegree C)(5 \textdegree C)\)
Multiply the first 2 numbers together. The units of grams cancel.
\(q= (387 \ J/ \textdegree C )(5 \textdegree C)\)
Multiply again. This time the units of degrees Celsius cancel.
\(q= 1935 \ J\)
1935 Joules of energy are required and choice C is correct.
please help with this greatly appreciated





Answers
It is true that according to Hubble's law, the farther away a galaxy is, the faster it is moving away from us.
It is true that the formation of a star occurs when nuclear fusion begins to fuse light elements into heavier ones;
The distance to the nearest stars can be determined by parallax, the apparent shift of a start against background stars observed as the earth moves along its orbit. (Option B)
Based on the accompanying H-R Diagram, the type of start that has the greatest temperature is the blue giants (Option C)
Two hydrogen atoms come together in a nuclear fusion reaction to produce Helium Gas. (Option A).
What is Hubble's Law?Hubble's law, which essentially states that the velocity of a galaxy (or, as it is commonly plotted, its redshift) is precisely proportionate to its distance, also reveals crucial information about the condition of the universe. There should be no relationship between distance and velocity if the cosmos is static and unchanging.
Hubble's law is the physical cosmology observation that galaxies move away from Earth at a rate proportionate to their distance. In other words, the farther they are from Earth, the quicker they are travelling away.
Learn more about Hubble Law:
https://brainly.com/question/13705068
#SPJ1
you have a sodium hydroxide solution that is approximately 0.2 m that you need to standardize. in order to make efficient use of your 50 ml buret, you need to know how many runs you can perform. estimate how many mls of 0.2 m naoh will be ne
Answers
4ml of NaOH is required for neutralization of KHP.
The chemical reaction is as follows:
NaOH + КНР → NaKP + H₂O
The mass of KHP used is 0.1509g, Molar mass of KHP is 204.29g/mol
Then moles of KHP used are given by
Moles of KHP used = 0.1509/204.29
Moles of KHP = 7.35 x 10⁻⁴ moles
The molarity of NaOH is 0.2 M Let the volume of NaOH required for neutralization be V.
Moles of NaOH needed = Molarity x volume of NaOH (L)
= 0.2 x V
As 1 mole of NaOH requires 1 mole of KHP, then
Moles of NaOH required = Molarity of NaOH x volume of NaOH (L)
On substituting the values we get,
0.2 V = 7.35×10⁻⁴
V = 7.35x10⁻⁴/0.2
= 3.675 X 10⁻³L
V = 3.675 mL
≈ 4mL
Therefore, 4 ml of NaOH is required for neutralization.
To know more about neutralization, click below:
https://brainly.com/question/23008798
#SPJ4
Why are the non polar compounds are insolube in water?
Answers
Due to the absence of polar functional groups and the inability to hydrogen bind with water molecules, nonpolar substances cannot dissolve in water.
Water is a polar solvent because it has a positive charge (hydrogen) on one end and a negative charge (oxygen) on the other (oxygen). Water's polarity makes it possible for it to establish hydrogen bonds with other polar compounds like salts and carbohydrates. Contrarily, nonpolar molecules lack polar functional groups and can't establish hydrogen bonds with water because they can't. As a result, nonpolar substances frequently have a low solubility in water and prefer to dissolve in other nonpolar solvents like benzene or hexane.Because nonpolar chemicals and water do not form hydrogen bonds, there is a strong attraction between the molecules of the nonpolar compound and water, causing the two substances to separate into discrete layers.
Learn more about polar here:
https://brainly.com/question/30002497
#SPJ4
what happens to the speed of a sound wave from an underwater animal as the sound passes into the air above?
A. It stays the same
B. It falls to zero
C. It decreases
D. It increases

Answers
i believe so.
https://www.change.org/p/scholastic-bring-back-maya-and-miguel/dashboard?source_location=user_profile_started
SIGN PLZ TO BRING BACK MY CHILDHOOD SHOW
Answers
Answer:
I got it
Explanation:
Thank you friend
Answer:Ok I got it, thanks for the score
Explanation:Have a nice day
someone help me with this problem pls

Answers
Answer:
magnetic field
Explanation:
What type of reaction does this model represent?
1. single replacement
2. double replacement
3. Decomposition
4. double displacement

Answers
Answer:
1. single replacement
Explanation:
Single replacement can be represented by AB+C ⇄B+AC, which matches the picture
Double replacement and double displacement are the same kind of reaction and can be represented by AB+CD ⇄BD+AC
Decomposition reaction can be represented by AB ⇄A+B
The respiratory system is what brings in food and breaks it down True or false
Answers
Answer:
false
Explanation:
the respiratory system includes the lungs and heart
not food
describe the Solvay process
Answers
Which statement describes the moment magnitude scale? Sorry if problemy not chemistry.
Answers
Answer:
Explanation:
The moment magnitude scale is a scale that rates earthquakes by estimating the total energy released by an earthquake . Estimating the total amount of energy released, enables comparison of earthquakes more accurately
i hope this helps :)
Dry suits made from tri-butyl laminate material are composed of alternate layers of nylon and butyl rubber. These suits offer a good combination of:
Answers
Dry suits made from tri-butyl laminate material offer a good combination of durability, flexibility, and waterproofness.
What are the key advantages of tri-butyl laminate dry suits?Tri-butyl laminate dry suits are constructed with alternating layers of nylon and butyl rubber. This design provides several benefits. Firstly, the use of nylon enhances the durability of the suit, making it resistant to tears and abrasions.
The butyl rubber layers contribute to the flexibility of the suit, allowing for ease of movement and comfort during use. Additionally, the combination of nylon and butyl rubber creates a waterproof barrier, keeping the wearer dry even in wet conditions.
This makes tri-butyl laminate dry suits suitable for activities such as diving, water rescue, and marine research, where protection from water exposure is crucial.
Tri-butyl laminate dry suits are specifically engineered for water-based activities that require protection against water ingress. The incorporation of alternating layers of nylon and butyl rubber creates a strong and flexible material that can withstand harsh environmental conditions.
The nylon provides strength and resistance to tearing, while the butyl rubber ensures a waterproof seal. These suits are commonly used in various water sports, diving, and other professions that involve working in or around water.
The combination of durability, flexibility, and waterproofness offered by tri-butyl laminate dry suits makes them a reliable choice for individuals seeking reliable protection and performance in wet environments.
Learn more about dry suits
brainly.com/question/32224564
#SPJ11
if 1500 tons of ammonia is needed to be produced daily to meet the needs of industry, what mass of nitrogen must be used from the atmosphere
Answers
if 1500 tons of ammonia is needed to be produced daily to meet the needs of industry mass of nitrogen must be used from the atmosphere is 6.291 gram
Ammonia has alkaline properties and is corrosive and ammonia gas dissolves easily in water to form ammonium hydroxide a caustic solution and weak base
Here given data is 1500 tons of ammonia
1 tons = 1,000,000gram so
1500 tons = 1,500,000,000 gram
We have to calculate mass of nitrogen in 1500 tons ammonia =?
So, 1500 tons/17.031g/mol = 88.074g/mol
Then 88.074g/mol/14g/mol
= 6.291 gram
Therefore mass of nitrogen must be used from the atmosphere is 6.291 gram
Know more about nitrogen
https://brainly.com/question/14357532
#SPJ1
How many grams of nitric acid are produced from this reaction if it reduces the partial pressure of no2 from a 1. 3×109l volume of air over a city by 4. 5×10−8atm when the temperature is 10∘c?
Answers
\(NO_{2}\)(g) + OH(g) = \(HNO_{3}\) (aq) (nitric acid)
Given,
Pressure of \(NO_{2}\) = 4.5x10-8 atm,
volume = 1.3x109 L, and
temp = 10ºC + 273 = 283K
moles. NO2 =? = n
PV = nRT
n = PV/RT = (4.5x10-8 atm)(1.3x109 L) / (0.0821 Latm/Kmol) (210K)
n = 3.4x10-18 moles NO2 = mols HNO3
mass HNO3 = 3.4 x 10 18 moles x 31 g/mol = 1.05 x 10 16 g.
Nitric acidThe inorganic substance nitric acid has the formula HNO3. This mineral acid is extremely corrosive. [5] Even though the chemical is colorless, older samples often have a yellow tinge from breakdown into nitrogen oxides. Nitric acid is 68 percent concentrated in water in the majority of commercially available products. Fumigating nitric acid is the term used to describe a solution that contains more than 86 percent HNO3. Depending on the quantity of nitrogen dioxide, fuming nitric acid is further classified as red fuming nitric acid at concentrations above 86 percent or white fuming nitric acid at concentrations above 95 percent. The main chemical used in nitration, or adding a nitro group usually to an organic molecule, is nitric acid.
How many grams of nitric acid are produced from this reaction if it reduces the partial pressure of no2 from a 1. 3×109l volume of air over a city by 4. 5×10−8atm when the temperature is 10∘c?
Learn more about nitric acid here:
https://brainly.com/question/26015251
#SPJ4
Why is melting a physical property of the wax, while flammability is a chemical property of the wax?.
Answers
Answer:
because melted wax is still wax and flammability is just a chemical property
Explanation:
GUYS HELP PLS QUICK LOTS OF POINTS AND BRAINLIEST!
A 0.250 M solution was prepared from 135 mL of a 7.00 solution. What volume of water (in mL) was required for dilution?
Answers
Answer:
385
Explanation:
0.250m Converted to 250 mL then 250+135+ the answer
I'm not sure?
what happens when throw up then eat it again?
Answers
Which of the physical states of water contains particles with the highest kinetic energy?
a. Water
b. Melting Ice
c. Steam
d. Ice