What is the common name for aspirin?
Answers
Answer:acid
Explanation:
The common name for aspirin is Acetylsalicylic acid (acetosal).
What is aspirin ?
Only those who have taken this medication can be discovered with acetylsalicylic acid (acetosal), also known as aspirin. The medication acetylsalicylic acid belongs to the salicylate family and is frequently used as an analgesic (to treat minor aches and pains), antipyretic (to treat fever), and anti-inflammatory.
What are the uses of aspirin?
Aspirin is used to treat a variety of ailments, including the common cold, headaches, toothaches, and mild to moderate muscle and joint pain. In diseases like arthritis, it can also be used to lessen discomfort and swelling.
Therefore, common name for aspirin is Acetylsalicylic acid (acetosal).
Learn more about aspirin from the given link.
https://brainly.com/question/25794846
#SPJ4
Related Questions
How many moles are present in 2.45×10²³ molecules of CH4 ? Show your work!!!
Answers
The number of moles of CH4 present in 2.45 × 10²³ molecules is 0.41 moles.
How to calculate number of moles?The number of moles of a substance can be calculated by dividing the number of molecules by Avogadro's number.
no. of moles = no of molecules ÷ 6.02 × 10²³
According to this question, 2.45×10²³ molecules of CH4 are given. The number of moles is calculated as follows:
no of moles = 2.45 × 10²³ ÷ 6.02 × 10²³
no of moles = 2.45/6.02
no of moles = 0.41moles
Therefore, the number of moles of CH4 present in 2.45 × 10²³ molecules is 0.41 moles.
Learn more about number of moles at: https://brainly.com/question/21494857
Calculate the acceleration of Josh riding his bicycle in a straight line that speeds up from 4 m/s to 6 m/s in 5 seconds. Initial Speed ____. Final Speed ______. Time ______. Acceleration =________
Answers
Answer:
Initial Speed ____4m/s. Final Speed ______6m/s. Time ______5 seconds . Acceleration =________0.4m/s²
Explanation:
The acceleration is the rate of change of velocity during time t .
acceleration = Final velocity- Initial velocity/ Time
= Vf- Vi/ t
a = Δv/ Δt
= 6m/s- 4m/s/ 5s
= 2m/s/ 5s
= 0.4m/s²
It can also be defined as the difference of the initial and final velocity ( velocities measured in meters per second) during the given time t measured in seconds .The SI unit of acceleration is meter per second squared.
If the velocities are not measured in meters per second or time is not measured in seconds then the units are changed accordingly.
Which of the following molecules would have the highest boiling point?
a) hexane
b) octane
c) 2-propylpentane
d) 2-methylhexane
Answers
The molecule which would have the highest boiling point is 2-methylhexane. Thus, the correct option will be D.
What is boiling point?The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point of a liquid is a measure of its vapor pressure. The higher the boiling point, the higher the vapor pressure of the liquid, and the more heat is required to vaporize it.
The boiling point of a substance is affected by the strength and types of intermolecular forces. The stronger the intermolecular forces, the higher the boiling point. 2-methylhexane has highest boiling point because it has the highest number of carbons and branches, which contribute to its strong intermolecular forces that lead to a higher boiling point.
Therefore, the correct option is D.
Learn more about Boiling point here:
https://brainly.com/question/25777663
#SPJ11
What is DNA fingerprinting?
FORENSICS
Answers
Answer:
It is a method used to identify a suspect.
Explanation:
DNA fingerprinting is a method used to identify an individual from a sample of DNA by looking at unique patterns in their DNA.
Complete and balance the following equations representing neutralization reactions: 28. 2CsOH + H2CO3 ?--+- 29, 2HF + Mg(OH)2 ?--+- 30. 3HNOg + Al (OH)3?--+- 31, + ?H2O + FrF 32 + ?H2O + LiBrOg
Answers
The neutralization reactions can be completed and balanced as follows:
28. 2CsOH + H2CO3 → Cs2CO3 + 2H2O
29. 2HF + Mg(OH)2 → MgF2 + 2H2O
30. 3HNO3 + Al(OH)3 → Al(NO3)3 + 3H2O
31. H2O + FrF → FrOH + HF
32. H2O + LiBrO → LiOH + HBrO
28. The neutralization reaction between CsOH (cesium hydroxide) and H2CO3 (carbonic acid) results in the formation of Cs2CO3 (cesium carbonate) and 2H2O (water). The balanced equation is: 2CsOH + H2CO3 → Cs2CO3 + 2H2O.
29. The neutralization reaction between HF (hydrofluoric acid) and Mg(OH)2 (magnesium hydroxide) produces MgF2 (magnesium fluoride) and 2H2O (water). The balanced equation is: 2HF + Mg(OH)2 → MgF2 + 2H2O.
30. The neutralization reaction between HNO3 (nitric acid) and Al(OH)3 (aluminum hydroxide) yields Al(NO3)3 (aluminum nitrate) and 3H2O (water). The balanced equation is: 3HNO3 + Al(OH)3 → Al(NO3)3 + 3H2O.
31. The reaction between H2O (water) and FrF (francium fluoride) results in the formation of FrOH (francium hydroxide) and HF (hydrofluoric acid). The balanced equation is: H2O + FrF → FrOH + HF.
32. The neutralization reaction between H2O (water) and LiBrO (lithium hypobromite) forms LiOH (lithium hydroxide) and HBrO (hypobromous acid). The balanced equation is: H2O + LiBrO → LiOH + HBrO.
To learn more about neutralization reactions, refer:-
https://brainly.com/question/27745033
#SPJ11
Of the following, which can be used to identify an element present in a sample?
Answers
Answer:
idk but i tryed
Explanation:
The simplest way to use the periodic table to identify an element is by looking for the element’s name or elemental symbol. The periodic table can be used to identify an element by looking for the element’s atomic number. The atomic number of an element is the number of protons found within the atoms of that element.
If 359 mL of a gas were contracted to only 269 mL, what would be the initial
temperature of it if it measured 422 K after the change?
Answers
A gas that initially had a temperature of 422 K and was reduced to only 269 mL would now have an initial temperature of 563.19 K.
Volume at start: 359 mL
Volume completed: 269 mL
422 K is the final temperature.
Temperature at the start =?
Charle's Law can be used to resolve the issue. Charles Law states that with constant pressure and molecular number, the volume of a given amount of a gas is directly proportional to its temperature.
V₁/T₁ = V₂/T₂
Initial volume: V1
Initial temperature is T1.
Final volume is V2.
T2 = Actual temperature.
We will now enter the values into the formula.
V₁/T₁ = V₂/T₂
T₁ = V₁T₂ / V₂
T1 = 359 ml 269 ml / 422 K
T1=151498 ml.K/ 269 ml
T₁ = 563.19 K
To know more about Charle's law visit
https://brainly.com/question/16927784
#SPJ4
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
What is a reaction rate?
Answers
Answer:
Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. It is often expressed in terms of either the concentration (amount per unit volume)
Explanation:
Answer:
A measure of how fast products are made in a reaction.
Explanation:
i just took the test and it gave me this cuz the other person was wrong
No underwater luminaires shall be installed in a permanently-installed swimming pool that operates on supply circuits over ? between conductors.
Answers
No luminaires shall be installed for operation on supply circuits over 150 volts between conductors. Luminaires mounted in walls shall be installed with the top of the luminaire lens not less than 450 mm (18 in.)
In an electrical installation, what is a luminaire?
A luminaire, also known as a light fixture, is a complete lighting assembly that includes one or more lamps (light bulbs or tubes), a socket and other protective elements, wiring that links the lamp to a power source, and a reflector to help direct and spread the light.
The construction, installation, or inclusion of shades or guards on luminaires is required to prevent the exposure of combustible materials to temperatures above 90°C (194°F). Unswitched lamp holders must be used above materials that are very flammable.
Learn more about luminaire from
brainly.com/question/4333610
#SPJ4
Why does a hot-air-balloon rise when the air inside is heated? Btw I'm learning about things like Charles Law, Avogadro, etc if that helps.
Answers
When heated, the air in the balloon will expand, resulting in an increase in volume and a reduction in density. The air within the balloon will thus be lighter than the air outside. The balloon then rises higher as a result.
How does Charles Law explain the reason that hot-air balloons float?Charles discovered that a gas's volume and temperature are inversely correlated when working with balloons. This link between temperature and gas volume, which came to be known as Charles' law, explains how hot-air balloons function.
The outcome is an increase in gas volume in line with Charles' law. As the balloon expands, the air within gets less dense and lifts the balloon.
learn more about Charles' law
https://brainly.com/question/16927784
#SPJ1
help please don’t understand

Answers
hello sister the answer is very easy it is 2 miles of oxygen
3-8 find the exact value of each expression.
5. (b) \(\log _{10} 0.0001\)
Answers
the exact value of the expression \(\log _{10} 0.0001\) is -4.
The expression given is \(\log _{10} 0.0001\).
We know that \(\log _{10} 0.0001\) is the power to which 10 must be raised to get 0.0001.
We can write 0.0001 as 1/10,000 or in exponential form, 10^-4.
Therefore, \(\log _{10} 0.0001 = \log _{10} 10^{-4}\).
Using the property of logarithms, \(\log _{a}a^{b}=b\), we get
\(\log _{10} 10^{-4} = -4\).
So, the exact value of the expression \(\log _{10} 0.0001\) is -4.
the exact value of the given expression is -
Expression: \(\log _{10} 0.0001\)
Recall that the logarithm is the inverse of exponentiation. In this case, we want to find the exponent to which 10 must be raised to obtain 0.0001.
Since 0.0001 = 10^(-4), the expression simplifies as follows:
\(\log _{10} 0.0001 = \log _{10} 10^{-4}\)
By the property of logarithms, the exponent can be directly extracted when the base of the logarithm matches the base of the exponent:
\(\log _{10} 10^{-4} = -4\)
So the exact value of the expression is -4.
To know more about exponent visit:
https://brainly.com/question/5497425
#SPJ11
Calculate the number of moles when 3.00l of a gas at 814 mmhg and 25 °c?
Answers
So
No of moles=n
\(\\ \rm\rightarrowtail PV=nRT\)
\(\\ \rm\rightarrowtail n=\dfrac{PV}{RT}\)
\(\\ \rm\rightarrowtail n=\dfrac{814(3)}{25R}\)
\(\\ \rm\rightarrowtail n=2442/25R\)
\(\\ \rm\rightarrowtail n=97.7Rmol\)
13 : 22
MI SEC
23. Which of the following solutions will reach dynamic equilibrium?
a Supersaturated solution
b Unsaturated solution
C Saturated solution
d None of the above
Answers
Answer:
C
Explanation:
The solution that will reach dynamic equilibrium is a saturated solution. The correct option is C.
What is an unsaturated solution?A chemical solution in which the solute concentration is less than its equilibrium solubility is known as an unsaturated solution.
The solvent dissolves the entire solute.
When a solute (typically a solid) is mixed with a solvent (commonly a liquid), two reactions take place at the same time.
Thus, the correct option is C, the saturated solution.
Learn more about saturated solutions.
https://brainly.com/question/1851822
What is the ph of a solution which results from mixing 25.0 ml of 0.300 m nh3 and 50.0 ml of 0.100 m hno3? the pka for nh4 is 9.24.
Answers
The pH of the solution resulting from mixing 25.0 mL of 0.300 M NH₃ and 50.0 mL of 0.100 M HNO₃ is approximately 4.83.
To find the pH of the solution, we need to determine if the resulting solution is acidic, basic, or neutral. Given that NH₃ is a weak base and HNO₃ is a strong acid, the NH₃ will act as the base and the HNO₃ will act as the acid.
First, we need to calculate the number of moles of NH₃ and HNO₃ that are present in the solution.
For NH₃ :
moles of NH₃ = volume of NH3 (L) × concentration of NH3 (mol/L)
moles of NH₃ = 0.025 L × 0.300 mol/L
moles of NH₃ = 0.0075 mol
Next, we need to determine which species will be present in excess. Since NH₃ has a higher number of moles, it will be the limiting reagent.
The reaction between NH₃ and HNO₃ will result in the formation of NH4+ and NO3-. The NH4+ will determine the pH of the solution.
To calculate the concentration of NH4+ in the solution, we need to determine the concentration of that reacted with HNO₃ . This can be done by subtracting the moles of that reacted from the total moles .
moles of NH3 remaining = moles of NH3 initially - moles of NH3 reacted
moles of NH3 remaining = 0.0075 mol - 0.005 mol
moles of NH3 remaining = 0.0025 mol
Therefore, the pH of the solution resulting from mixing 25.0 mL of 0.300 M NH3 and 50.0 mL of 0.100 M HNO3 is approximately 4.83.
learn more about strong acid
https://brainly.com/question/24586675
#SPJ11
Can someone help me

Answers
Answer:the second largest one
Explanation:
both weels are pushing at it
Please help I’m horrible at this and can’t figure it out anywhere

Answers
Answer:
I believe it is C.
Explanation:
Everything else is true.
Electromagnetic waves do not need an medium to travel though.
Answer:
C
Explanation:
EM waves need a medium mechanical waves don't
Aqueous ammonia is commercially available in a solution that is 28% (w/w) ammonia. What is the mole fraction of ammonia in such a solution?.
Answers
The the mole fraction of ammonia in such a solution is 0.291.
What is aqueous solution?
Water that has one or more dissolved substances in it is called an aqueous solution. An aqueous solution can contain particles, gases, or other liquids that have been dissolved in it. A mixture must be steady in order to be a real solution.
What is mole fraction?
By dividing the total number of moles in the given mixture by the number of molecules of a certain component, the term "mole fraction" is used. The concentration of a solution is expressed in this fraction.
28 % w/w means 28 gms ammonia in 100 gms ammonia solution
so water = 100-28 = 72 gms
moles of NH3 = 28/17 = 1.647
moles of water = 72/18 = 4
totals moles = 4+1.647 = 5.647
mole fraction of ammonia = moles of NH3/ total moles
= 1.647 /5.647 = 0.291
Therefore, the mole fraction of ammonia in such a solution is 0.291.
Learn more about aqueous solution from the given link.
https://brainly.com/question/19587902
#SPJ4
what energy storage molecule is produced when food is broken down during the cellular respiration process?
Answers
The energy storage molecule that is produced when food is broken down during the cellular respiration process is ATP molecule.
The Cellular respiration is a metabolic pathway that generally breaks down glucose molecules and produces ATP. The stages of cellular respiration basically includes glycolysis, pyruvate oxidation, the citric acid or Krebs cycle, and oxidative phosphorylation etc.
Cell respiration usually consists of three steps that is glycolysis, the Krebs cycle, and the respiratory electron transport. The first step by which cells make the ATP from food is called glycolysis. For example Imagine eating a donut. The starches and sugars of the donut are generally converted to glucose that is blood sugar, in your mouth and stomach by digestive the enzymes.
During the cellular respiration, glucose is usually broken down in the presence of oxygen in order to produce carbon dioxide and water. Energy released during the reaction is usually captured by the energy-carrying molecule ATP (adenosine triphosphate).
To know more about cellular respiration process
https://brainly.com/question/29760658
#SPJ4
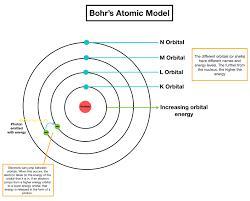
Why is electron filling similar to going up the stairs?.
Answers
If we look into the Bohr's atomic model, it is like a staircase.
Where every next orbit is like a stair.
As we can not rest in air, we either have to stay on level 1 or can go up with enough energy to climb next level.
Same way, electron can either stay on one orbit or can go to higher orbits with enough energy to jump from lower energy level to the higher energy level, can say orbit.
According to Bohr, atom is a system consisting of a dense nucleus surrounded by orbiting electrons.
He compared his atomic structure with the structure of the Solar System.
To know more about Bohr's atomic model:
brainly.com/question/3964366
#SPJ4
Calculate the following examples.
1. Ramji conducted an experiment in the field related to the rate of percolation. He observed that it took 30 minutes for 600 ml of water to percolate through the soil. Calculate the rate of percolation per hour.
2. Jigna takes 50g of dry powdered soil in a filter paper and slowly adds water to the soil from a 100 ml fully filled measuring cylinder. When water starts dripping from the filter paper, 60 ml of water is left in the measuring cylinder. Find out the percentage of water absorbed by the soil.
Answers
Answer:
12ml per hour and
69% LOL
1) The rate of percolation would be 0.33 ml/s
Percolation rate = volume of water/time
= 600/1800s
= 0.33 ml/s
2) The percentage of water absorbed would be 40%
Percentage of water absorbed = amount absorbed/total water x 100
amount of water absorbed = 100 - 60
= 40 ml
total amount of water = 100 ml
Percentage of water absorbed = 40/100 x 100
= 40%
More on numerical calculations can be found here; https://brainly.com/question/24638543?referrer=searchResults
True or False questions
• Please answer the following questions
1. Water is released from plants & animals
False
—
2.
Water contains 2 atoms or oxygen
3.
Water is found in plants but not in meats
4.
Water can dissolve many substances
Answers
Answer:
I did not understand the question
3. Humans discovered how to use fire and tools. How was this knowledge passed along to younger generations?
Communication skills were developed so knowledge could be passed along to younger generations.
Primitive knowledge was instinctive so that the basic skills of fire and tools came naturally to everyone.
Younger people were forced to spend long hours building fires and making tools.
O The younger generations were able to figure out how to use tools on their own.
Answers
Communication skills were developed so knowledge could be passed along to younger generations.
How knowledge will pass through generations?Knowledge will pass from one generation to another with the help of education. The skill people give education to their youngers about fire and tools. In this way, skills of fire and tools pass from one generation to another.
So we can conclude that communication skills were developed so knowledge could be passed along to younger generations.
Learn more about knowledge here: https://brainly.com/question/24621985
#SPJ1
Answer:
A
Explanation:
what is the sign of for the following processes? people leaving a classroom and going to different classrooms stacking sheets of paper [ select ] dissolving salt in water [ select ] burning sugar [ select ] moving a pencil from one side of a desk to another
Answers
People leaving a classroom and going to different classrooms: This is a process of diffusion, where particles move from an area of high concentration to an area of low concentration. The sign for this process is negative because it results in a decrease in the concentration of particles in the initial area.
Stacking sheets of paper: This is a process of increasing order or organization, and it does not involve any significant change in energy. Therefore, the sign for this process is neither positive nor negative.
Dissolving salt in water: This is a process of dissolution, where salt crystals break down into individual ions and become surrounded by water molecules. This process involves the transfer of energy between the system and the surroundings. The sign for this process is negative because it releases energy to the surroundings.
Burning sugar: This is a process of combustion, where the sugar molecules react with oxygen in the air to produce carbon dioxide and water, releasing a large amount of energy. The sign for this process is highly positive because it involves a significant increase in energy as the bonds in the sugar molecules are broken and new bonds are formed.
Moving a pencil from one side of a desk to another: This is a process of physical movement that does not involve any significant change in energy. Therefore, the sign for this process is neither positive nor negative.
To learn more about water refer to:
brainly.com/question/1216922
#SPJ4
A titration of 200.0 mL of 1.00 M H2A was done with 1.38 M NaOH. For the diprotic acid H2A, Ka1 = 2.5 10–5, Ka2 = 3.1 10–9. Calculate the pH after 100.0 mL of 1.38 M NaOH have been added.
Answers
Answer:
4.95
Explanation:
1.00 M H2A
1.38 m NaOH
Titration = 200.0 mL
Calculate moles of NaOH
= \(\frac{100*1.38}{300}\) = 0.46
calculate moles of H2A
= \(\frac{200 * 1.0}{300}\) = 0.667
therefore the moles of acid left = moles of H2A - moles of NaOH
= 0.667 - 0.46 = 0.207
pka = - log( ka )
= - log ( 2.5 * 10^-5 ) = 4.61
calculate PH after 100 ml of 1.38 M NaOH have been added
PH = pka + log \((\frac{salt}{acid} )\)
= 4.61 + log \((\frac{0.46}{0.207} )\) = 4.95
The Statue of Liberty is made out of copper and was once shiny and copper-colored. Over the years, the copper has gone through a process called oxidation, where it has changed into a new material. What has occurred? an endothermic reaction an energy formation a chemical reaction a precipitate formation
Answers
Answer:
C. A chemical reaction.
Explanation:
A chemical reaction can be defined as a chemical process which typically involves the transformation or rearrangement of the atomic, ionic or molecular structure of an element through the breakdown and formation of chemical bonds to produce a new compound or substance.
Since the Statue of Liberty is made out of copper and was once shiny and copper-colored. Over the years, the copper has gone through a process called oxidation, where it has changed into a new material. Thus, this is a chemical reaction.
In this case, occurred a CHEMICAL REACTION known as oxidation.
What is a chemical reaction?A chemical reaction is a process where one or more substances called reactants can react to be converted into one or more products.
The process of oxidation is a type of chemical reaction where a specific element/atom (i.e., the reactant) loses its electrons. This chemical reaction (oxidation) is always combined with another chemical reaction known as 'reduction' in which an element/atom gains electrons.In conclusion, in this case, occurred a CHEMICAL REACTION known as oxidation.
Learn more about chemical reactions here:
https://brainly.com/question/11231920
What nuclear reaction could hydrogen undergo, fission or fusion?*
Answers
Answer:
Hydrogen (H) “burning” initiates the fusion energy source of stars and leads to the formation of helium (He).
FUSION Reaction
Explanation:
mark me brainliest please.
of the 20 standard amino acids, only is not optically active. the reason is that its side chain .
Answers
Glycine is the only amino acid that is not optically active because it does not have a carbon chiral center.
The simplest amino acid is glycine. Because it is the only amino acid without an asymmetrical carbon atom, it differs from all the other amino acids. Glycine is therefore optically inactive. No stereoisomers exist in it. This amino acid is necessary for the manufacture of bile acids, porphyrins, creatine phosphate, and other amino acids as well as nucleic acids.
When compared to the other amino acids, glycine is integrated into proteins and enzymes at a rate of 7.5%, making it the second most prevalent amino acid on a molar basis. Glycine shares a similar ability to block neurotransmitter impulses in the central nervous system with gamma-aminobutyric acid and glutamic acid.
Learn more about glycine here:
https://brainly.com/question/28146647
#SPJ4
Which two of the minerals shown have a metallic luster?

Answers
Answer:
A and D
Explanation:
Answer:
A & D
Explanation: