What is the method of cooking chicken by browning in hot fat and then covering and cooking in the oven?
Answers
Poêléing is the process of frying chicken in hot fat before wrapping it and baking it.
What is Poêléing?
In the dry-heat cooking technique known as poêléing, the food is cooked in its own juices in a covered pot, typically in the oven.
Poêléing is often referred to as butter roasting.
To know more about Poêléing go to the given link:
https://brainly.com/question/4738210
#SPJ4
Related Questions
Classify the descriptions of keratin, collagen, and fibroin. Some phrases may apply to more than one protein. a-Keratin Collagen Fibroin Collagen and fibroin Answer Bank contains hydroxyproline every third residue is glycine contains heptad repcals has intrachain hydrogen bonds has left-handed helices has interchain hydrogen bonds every second residue is glycine
Answers
a-Keratin has heptad repeats, has intrachain hydrogen bonds, has left-handed helices.
Collagen contains hydroxyproline, every third residue is glycine, has interchain hydrogen bonds.
Fibroin every second residue is glycine.
What is the role of a-Keratin?Alpha-keratin is a fibrous protein that is the main component of hair, nails, horns, claws, and hooves of animals. Its role is to provide structure, strength, and resilience to these tissues, which need to withstand mechanical stress and damage.
Alpha-keratin is particularly abundant in hair, where it forms the hair shaft's outer layer, or cuticle. Its structure includes an alpha-helix conformation, stabilized by hydrogen bonds between the peptide backbone's oxygen and hydrogen atoms.
Learn more on a-Keratin here: https://brainly.com/question/10703381
#SPJ1
Just need to make sure if this is right
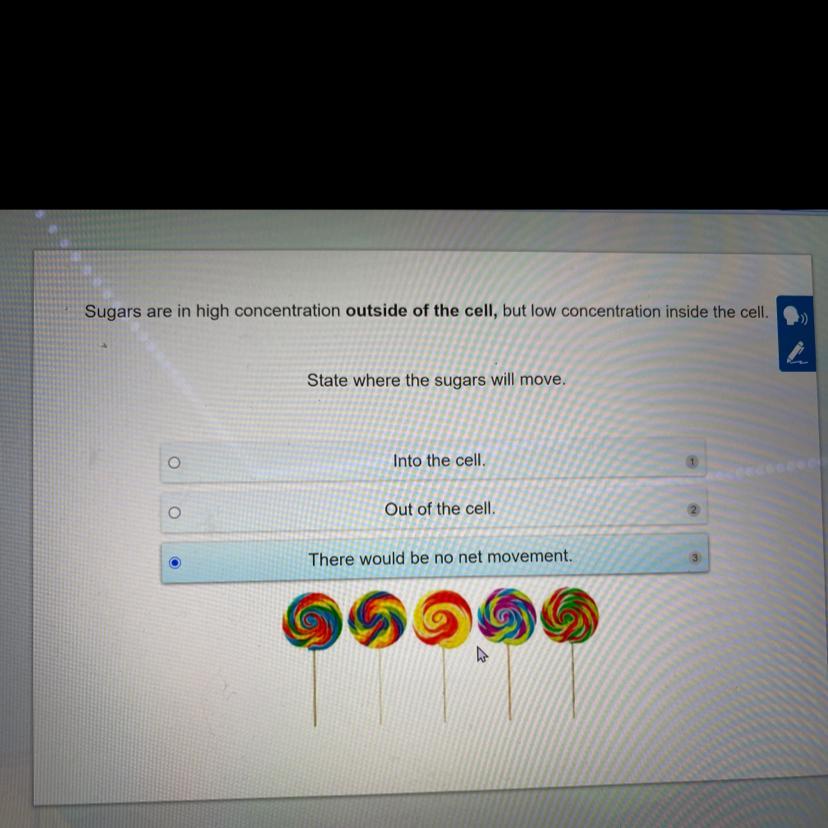
Answers
Answer:
yeah think that’s correct
Explanation:
not exactly sure how to explain this, I just know that it’ll prolly just stay the same and not move to any high and low regions of the cell
Which of the following best describes chicken noodle soup? * picture below.

Answers
Answer:
Chicken noodle soup would be considered a mixture.
What type of rock is this?

Answers
Answer:
I'm gonna say igneous
Explanation:
The texture looks rough and the rock looks like it's composed of different types of minerals making it an igneous rock
I hope this helps :)
Answer: Igneous
Explanation: Igneous rock, or magmatic rock, is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rock is formed through the cooling and solidification of magma or lava. The magma can be derived from partial melts of existing rocks in either a planet's mantle or crust.
Which are the products in the equation ch3sh 4o2 → co2 so2 2h2o? check all that apply. ch3sh o2 co2 so2 h2o
Answers
The products in the equation would be \(CO_2\), \(SO_2\), and \(H_2O\).
Chemical equationsFrom the equation of the reaction:
\(CH_3SH + 4O_2 --- > CO_2 + SO_2 +2H_2O\)
The products are \(CO_2\), \(SO_2\), and \(H_2O\).
This is because, for every chemical equation, the reactants are always at the back of arrows while products are at the front of arrows. In other words, reactants are usually on the left-hand side while products are on the right-hand side.
More on chemical reactions can be found here:https://brainly.com/question/22817140
#SPJ2
The atomic number identifies which of the following?
a
number of neutrons
b
number of electrons and neutrons
c
number of protons
d
number of protons and neutrons
Answers
Answer: protons
Explanation:
The atomic # identifies the amount of protons in an atom
What is the pH?
Show work
100. 0 mL of 0. 10 F H3PO4 is mixed with 200. 0 mL 0. 15 M NaOH.
250. 0 mL of 0. 10 M HA (Ka = 1. 0 x 10-4) is mixed with 100. 0 mL 0. 25 M KOH.
100. 0mLof0. 10MHA(Ka =1. 0x10-4)ismixedwith100. 0mLof 0. 050 M NaA
Answers
The pH of 100.0 mL of 0.10 M H₃PO₄ is mixed with 200.0 mL of 0.15 M NaOH is 1.00.
a) To determine the pH of the resulting solution, we need to calculate the concentration of the hydronium ion (H₃O⁺) using the concept of acid-base neutralization. The balanced equation for the reaction between H₃PO₄ and NaOH is:
H₃PO₄ + 3NaOH → Na₃PO₄ + 3H₂O
Since H₃PO₄ is a triprotic acid, we can assume that it completely dissociates in water. Therefore, the moles of H₃PO₄ can be calculated as follows:
Moles of H₃PO₄ = (0.10 mol/L) × (0.100 L) = 0.010 mol
To find the concentration of H₃O⁺, we need to consider the stoichiometry of the reaction. In the balanced equation, we see that for every mole of H₃PO₄, three moles of H₃O⁺ are produced. Therefore, the concentration of H₃O⁺ in the resulting solution is:
[H₃O⁺] = (3 × 0.010 mol) / (0.100 L + 0.200 L) = 0.030 mol / 0.300 L = 0.10 M
The pH can be calculated using the formula: pH = -log[H₃O⁺]
pH = -log(0.10) ≈ 1.00
Therefore, the pH of the resulting solution is approximately 1.00.
b) To determine the pH of the resulting solution, we need to consider the reaction between the weak acid (HA) and the strong base (KOH). The balanced equation for this reaction is:
HA + KOH → K⁺ + A⁻ + H₂O
Since HA is a weak acid, it will only partially dissociate in water. We need to consider the initial concentration of HA, the amount of KOH added, and the resulting volume of the solution.
First, let's calculate the moles of HA:
Moles of HA = (0.10 mol/L) × (0.250 L) = 0.025 mol
Next, let's calculate the moles of KOH:
Moles of KOH = (0.25 mol/L) × (0.100 L) = 0.025 mol
Since the moles of KOH are equal to the moles of HA, they will react completely in a 1:1 ratio, resulting in the formation of the potassium salt (K⁺A⁻) and water (H₂O).
The total volume of the resulting solution is the sum of the volumes of HA and KOH:
Total volume = 250.0 mL + 100.0 mL = 350.0 mL = 0.350 L
To determine the concentration of the resulting solution, we divide the moles of the species formed by the total volume:
Concentration of K⁺A⁻ = (0.025 mol) / (0.350 L) ≈ 0.0714 M
Since we have a salt in the solution, we can assume complete dissociation of the salt into its respective ions. Therefore, the concentration of the hydronium ion (H₃O⁺) will be equal to the concentration of the hydroxide ion (OH⁻) due to the neutralization reaction.
Now, let's calculate the concentration of H₃O⁺:
[H₃O⁺] = [OH⁻] = 0.0714 M
Finally, we can calculate the pH using the formula: pH = -log[H₃O⁺]:
pH = -log(0.0714) ≈ 1.15
Therefore, the pH of the resulting solution is approximately 1.15.
c) To determine the pH of the resulting solution, we need to consider the reaction between the weak acid (HA) and the weak base (A⁻). The balanced equation for this reaction is:
HA + A⁻ ⇌ H₂A
Since HA is a weak acid with a given Kₐ value, we can assume that it partially dissociates in water. The initial concentrations of HA and A⁻, as well as the resulting volume of the solution, are given.
First, let's calculate the moles of HA:
Moles of HA = (0.10 mol/L) × (0.100 L) = 0.010 mol
Next, let's calculate the moles of A⁻:
Moles of A⁻ = (0.050 mol/L) × (0.100 L) = 0.005 mol
Now, let's determine the concentrations of HA and A⁻ in the resulting solution:
[H₃A] = (moles of HA) / (total volume) = 0.010 mol / (0.100 L + 0.100 L) = 0.050 M
[HA] = (moles of A⁻) / (total volume) = 0.005 mol / (0.100 L + 0.100 L) = 0.025 M
Since HA and A⁻ have a 1:1 stoichiometric ratio, the concentrations of H₃A and HA are the same in the resulting solution.
To determine the concentration of the hydronium ion (H₃O⁺), we need to consider the dissociation of HA. The Kₐ expression for HA is:
Kₐ = [H₃O⁺] [A⁻] / [HA]
Given that Kₐ = 1.0 x 10⁻⁴ and the concentration of A⁻ is equal to the concentration of H₃A, we can rewrite the equation as:
(1.0 x 10⁻⁴) = (x)² / (0.025)
Solving for x (the concentration of H₃O⁺), we find:
x = √(1.0 x 10⁻⁴) × √(0.025) ≈ 0.0032 M
Now, we can calculate the pH using the formula: pH = -log[H₃O⁺]:
pH = -log(0.0032) ≈ 2.5
Therefore, the pH of the resulting solution is approximately 2.5.
The correct question is :
What is the pH?
100.0 mL of 0.10 M H₃PO₄ is mixed with 200.0 mL of 0.15 M NaOH.
250.0 mL of 0.10 M HA (Kₐ = 1. 0 x 10⁻⁴) is mixed with 100.0 mL of 0.25 M KOH.
100.0 mL of 0. 10 M HA (Kₐ =1. 0x10⁻⁴) is mixed with 100.0 mLof 0.050 M NaA
To know more about pH follow the link:
https://brainly.com/question/2288405
#SPJ4
I need this solved pls!!! Thanks! I know this is a lot, but can you solve at least one part?

Answers
The molar mass of calcium carbonate is 100 grams . This is determined by Stoichiometry.
What is molar mass ?The molar mass (M) of a chemical compound is defined in chemistry as the ratio of mass to substance (measured in moles) of any sample of said compound. The molar mass of a substance is a bulk property, not a molecular property. The molar mass is an average of many instances of the compound, which often vary in mass due to isotopes present. The molar mass is most commonly calculated from standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the constituent atoms on Earth. For bulk quantities, the molar mass is appropriate for converting between the mass of a substance and the amount of a substance.
In Calcium carbonate
mass of calcium = 40 g
mass of carbon = 12 g
mass of three oxygen atoms = 48 g
adding all molar mass = 100 g
to know more about molar mass , visit ;
brainly.com/question/22997914
#SPJ1
Electrolyte and water balance is facilitated through adequate intake of which of the following nutrients? A. iron, copper, zinc, iodine B. sodium, chloride, potassium, C. magnesium calcium, phosphorus, magnesium, manganese D. sulfur, selenium, fluoride
Answers
Electrolyte and water balance is facilitated through adequate intake of Sodium, Chloride, Potassium nutrients.
Option B is correct.
Which nutrients keep the electrolyte balance in check?Sodium, calcium, potassium, chloride, phosphate, and magnesium are electrolytes. You get them from the food sources you eat and the liquids you drink. It is possible for your body's electrolytes levels to become either too low or too high. This can happen when how much water in your body changes.
What does an electrolyte do?When dissolved in water, substances known as electrolytes naturally possess either a positive or negative electrical charge. They help your body control chemical reactions and keep the fluids inside and outside your cells in balance.
Learn more about Electrolytes:
brainly.com/question/17089766
#SPJ4
what is ions in chemistry?
Answers
In order to have a charge, an atom or molecule must have more electrons than protons, which is what creates an ion.
What substances make up ions?Uncharged atoms are referred to as ions (positive or negative). A material needs to gain or lose an electron in order to create an ion. Ions are created by either gaining or losing electrons. If an atom gains an electron, it will eventually have more electrons than protons, resulting in a negatively charged elemental atom overall.Any atom or group of atoms that has one or more positive or negative electrical charges is known as an ion. Cations are positive charges, while anions are negative charges, respectively.In order to have a charge, an atom or molecule must have more electrons than protons, which is what creates an ion.To learn more about ion refer to:
https://brainly.com/question/16934465
#SPJ1
Which of the following is an energy conservation practice?
Raise the temperature on a water heater.
Wash clothes in cold water instead of warm.
Run the washing machine with smaller loads.
Lower the thermostat's temperature in the summer.
Answers
Out of the options listed, washing clothes in cold water instead of warm is an energy conservation practice. Option C is correct.
Energy conservation refers to the practice of reducing the amount of energy used in daily activities while still achieving the desired results. This practice is essential in reducing greenhouse gas emissions, combating climate change, and reducing reliance on non-renewable energy sources.
This practice reduces the amount of energy required to heat water, which saves both energy and money. Heating water accounts for a significant amount of energy use in households, and by reducing the temperature, a significant amount of energy can be saved.
Raising the temperature on a water heater, on the other hand, is not an energy conservation practice. This action leads to increased energy consumption, as the water heater needs to work harder to maintain the desired temperature. It is recommended to keep the temperature of the water heater at 120°F to reduce energy consumption.
Running the washing machine with smaller loads can reduce water usage, but it may not necessarily reduce energy usage. Energy consumption is directly related to the amount of water used, the temperature of the water, and the duration of the washing cycle.
Lowering the thermostat's temperature in the summer can save energy if it leads to a reduction in air conditioning usage. However, if it leads to increased use of fans or other cooling equipment, it may not result in energy conservation.
In conclusion, washing clothes in cold water instead of warm is an energy conservation practice that households can adopt to reduce energy usage, save money, and contribute to environmental sustainability. Option C.
For more such questions on conservation visit:
https://brainly.com/question/14840218
#SPJ11
What is the final temperature of the water went 100 mL of 30°C water is mixed with 500 mL of 60°C water
Answers
The final temperature of the water resulting from the mixing of 100 mL of 30°C water with 500 mL of 60°C water would be 55°C.
Temperature calculationIn order to calculate the final temperature of a mixture of two different temperatures of water, we can use the following formula:
\(T_{(final)} = (m_1T_1 + m_2T_2) / (m_1 + m_2)\)
where:
T(final) is the final temperature of the mixturem1 and m2 are the masses of water in milliliters (mL) or grams (g)T1 and T2 are the initial temperatures of water in degrees Celsius (°C).In this case, we have 100 mL of 30°C water and 500 mL of 60°C water. We can convert mL to grams using the density of water which is approximately 1 g/mL2. Therefore:
m1 = 100 g T1 = 30°C m2 = 500 g T2 = 60°C
Thus:
T(final) = (100x30) + (500x60) / (100 + 500) T(final) = (3000 + 30000) / 600 T(final) = 55°CTherefore, the final temperature of the mixture is 55°C.
More on temperature can be found here: https://brainly.com/question/11464844
#SPJ1
what would you observe if sodium and potassium are placed in hot water.
Answers
Answer:
Sodium reacts more quickly, generating enough heat to melt itself and to occasionally ignite the hydrogen gas, producing a yellow-orange flame characteristic of sodium. The potassium reacts violently, immediately bursting into a flame which has the characteristic violet color of potassium.
Explanation:
What is an element? Arrow

Answers
Answer:
One arrow is positioned in each box according to Hund's Rule which tells us to maximise the number of unpaired electrons in orbitals of the same subshell, and, to give those electrons the same "spin" (parallel spin).
Explanation:
Cubes are three-dimensional square shapes that have equal sides. What is the density of a cube that has a mass of 12.6 g and a measured side length of 4.1 cm? (Density: D = )
Answers
Answer:
0.1828g/cm³
Explanation:
density= mass÷volume
m= 12.6g
v= 4.1×4.1×4.1 = 68.921cm³
•
density= 12.6÷68.921 = 0.1828g/cm³
Why is it important to have a specific system to measure when there is very little solute compared to the amount of solution(solvent)
Answers
When a solute dissolves, each of its atoms, molecules, or ions interacts with the solvent to become solvated and is then free to spread throughout the solution on their own.
Thus, However, this is not a one-way process. A process known as crystallization may occur if a molecule or ion happens to collide with the surface of an undissolved solute particles and solvent.
As long as there is surplus solid present, dissolution and crystallization proceed, creating a dynamic equilibrium similar to the equilibrium that maintains the vapour pressure of a liquid and solvent.
A solute's solubility is the greatest amount of that solute that can dissolve in a solvent at a given temperature and pressure. The moles of solute per volume (mol/L) or the mass of solute per mass of solvent (g/g) are other common ways to express solubility.
Learn more about Solubility, refer to the link:
https://brainly.com/question/31493083
#SPJ4
What mass of water is needed, so that when its temperature increases by 24.7°C, the heat energy is 24,183 J?
Answers
The mass of water needed to increase its temperature by 24.7°C and produce a heat energy of 24,183 J is 230.7 grams.
To calculate the mass of water required to increase its temperature by 24.7°C and produce a heat energy of 24,183 J, we can use the specific heat capacity of water. The specific heat capacity of water is 4.18 J/g°C. This means that it takes 4.18 joules of energy to raise the temperature of 1 gram of water by 1 degree Celsius.
Using this information, we can calculate the mass of water needed as follows:
Heat energy = mass x specific heat capacity x change in temperature
Substituting the values given in the question, we get:
24,183 J = mass x 4.18 J/g°C x 24.7°C
Simplifying this equation, we get:
mass = 24,183 J / (4.18 J/g°C x 24.7°C)
mass = 230.7 g
Therefore, the mass of water needed to increase its temperature by 24.7°C and produce a heat energy of 24,183 J is 230.7 grams.
To know more about Mass visit:
https://brainly.com/question/19694949
#SPJ11
you will ask questions about the factors that have caused a rise in global
temperatures over the past century
Answers
Answer: Air pollution is the main cause of rise in temperature over the past century.
Explanation:
Due to increase in the industrialization in different countries of the world and also burning of more fossil fuels in the engines of vehicles and jets are the main reason of increasing global temperature. Both industries and vehicles produce high amount of carbondioxide which is a greenhouse gas that blocks the passage of reflected radiation from the earth surface and traps this radiation which increases the temperature of the earth.
What is the relationship between electrons and elements with
negative oxidation numbers? between electrons and elements
with positive oxidation numbers?
Answers
Explanation:
The higher its electronegativity, the more an element attracts electrons. The atom with higher electronegativity, typically a nonmetallic element, is assigned a negative oxidation number, while metallic elements are typically assigned positive oxidation numbers.... Hope it is useful brainliest plz if it was useful....
The relationship between electrons and oxidation number has bee the negative oxidation number implies the gain of the electron, while positive oxidation number implies the loss of electron by elements.
The oxidation number has been defined as the number of electrons lost or gained by an element to form a chemical bond. The elements that lose electrons are assigned positive oxidation number.
The elements that gain electrons to form a chemical bond are assigned a negative oxidation number.
The relationship between electrons and oxidation number has bee the negative oxidation number implies the gain of the electron, while positive oxidation number implies the loss of electron by elements.
For more information about oxidation number, refer to the link:
https://brainly.com/question/10079361
two difference between simple cell and dry cell
Answers
Explanation:
Simple cell has liquid chemicals and it is difficult to carry from one place to another. Dry cell has no solution. So, it is easier to carry it from one place to another and there is no risk of spilling acid from the dry cell.
calculate the gibbs free energy of the following ethanol reaction. assume standard conditions (1 atm, 25oc).
Answers
The Gibbs free energy change (ΔG°) for the combustion of ethanol at standard conditions is -614 kJ/mol.
To calculate the Gibbs free energy change (ΔG°) for a reaction, we need the standard Gibbs free energy of formation (ΔG°f) values for the reactants and products involved in the reaction. The reaction you provided, the combustion of ethanol, can be represented as:
C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
The standard Gibbs free energy of formation values (ΔG°f) for the compounds involved are:
ΔG°f(C2H5OH(l)) = -174.8 kJ/mol
ΔG°f(O2(g)) = 0 kJ/mol
ΔG°f(CO2(g)) = -394.4 kJ/mol
ΔG°f(H2O(l)) = -237.2 kJ/mol
Now we can calculate the ΔG° for the reaction using the following equation:
ΔG° = ΣnΔG°f(products) - ΣnΔG°f(reactants)
where n is the stoichiometric coefficient of each compound.
For the given reaction:
ΔG° = (2ΔG°f(CO2(g)) + 3ΔG°f(H2O(l))) - (ΔG°f(C2H5OH(l)) + 3ΔG°f(O2(g)))
Plugging in the values:
ΔG° = (2(-394.4 kJ/mol) + 3(-237.2 kJ/mol)) - (-174.8 kJ/mol + 3(0 kJ/mol))
ΔG° = -788.8 kJ/mol - (-174.8 kJ/mol)
ΔG° = -614 kJ/mol
Therefore, the Gibbs free energy change (ΔG°) for the combustion of ethanol at standard conditions is -614 kJ/mol.
Learn more about ethanol here:
https://brainly.com/question/29294678
#SPJ11
Draw The Valence Bond Lewis Structure of Ne2^+2. Draw Molecular Orbital Diagram using Shorthand Notation. What is the bond order, number of sigma bonds, number of pi bonds? Is it paramagnetic?
Answers
magnetic quality Ne2 particle does not exist because the bond order is zero. Due to the lack of any unpaired electrons, it is diamagnetic. B2 compound.
A valence bond structure: what is it?VB Theory Valance Bond The valence bond hypothesis states that rather than occupying molecular orbitals, electrons in molecules do so in atomic orbitals. A chemical bond is created when two atomic orbitals overlap, and this overlapping causes the electrons to be concentrated in the bond region.
What does a Lewis structure bond look like?Using the model of localized electron bonds Two atoms sharing a pair of electrons results in bonds. Since an orbital may hold two electrons, we typically speak of bonding pairs and lone pairs of electrons.
To know more about Valance Bond visit :
https://brainly.com/question/866518
#SPJ4
Which is an empirical formula?
1.
H202
2.
H20
3.
C₂H₂
4.
C3H6
Answers
Answer:
c2h2 is it..........
Explanation:
lol
Sigmund freud's theories are considered both controversial and influential. based on what you learned in the lesson and your own personal experiences, do you agree with freud's theories
Answers
When considering Sigmund Freud's theories, it's important to acknowledge that they are both controversial and influential. Whether or not one agrees with Freud's theories depends on personal perspectives, experiences, and the specific theories being discussed.
1. Familiarize yourself with Freud's theories: Start by learning about the main ideas put forth by Freud, such as the unconscious mind, psychosexual stages of development, and defense mechanisms.
2. Consider the evidence: Evaluate the evidence supporting Freud's theories. This can involve reading research studies, scholarly articles, and critiques of his work. Assess the quality and validity of the evidence provided.
To know more about that perspectives visit:
https://brainly.com/question/11012390
#SPJ11
The pOH of a solution is 3.1. Which of the following is true about the solution? (1 point)
It is acidic and has a pH of 10.9.
It is basic and has a pH of 10.9.
It is acidic and has a pH of 6.2.
It is basic and has a pH of 6.2.
Answers
Answer:
The answer is option BExplanation:
To solve the question above we must first find the pH of the solution using the formula
pH + pOH = 14
pOH = 3.1
So we have
pH + 3.1 = 14
pH = 14 - 3.1
pH = 10.9
Since it's pH is 10.9 the solution is a basic solution since it's pH lies in the basic region.
Hope this helps you
What is the formula mass (molar mass) of cholesterol (C27H46O), a fat found in your blood and cell membranes? 19 g/mol 130 g/mol 260 g/mol 386 g/mol.
Answers
Answer:
386.66 g/mol
Explanation:
To calculate the molar mass of a molecule, you add up the molar masses of its atoms.
Thus, for cholesterol. we get
27C = 27 × 12.011 = 324.297 g
46H = 46 × 1.008 = 46.368
1O = 1 × 15.999 = 15.999
TOTAL = 386.655 g
To two decimal places, the molar mass of cholesterol is 386.66 g/mol
what is the sensitivity of the least sensitive balance most likely to be in your laboratory
Answers
The least sensitive balance in our laboratory is likely to have a sensitivity of around 0.1 grams. This balance is designed to measure relatively large quantities and is not suitable for precise measurements.
In our laboratory, we utilize a range of balances with varying sensitivities depending on the nature of the measurements required. The least sensitive balance is typically designed to handle larger quantities and is not intended for high-precision measurements. It is likely to have a sensitivity of around 0.1 grams, meaning it can detect differences in weight down to that level. This balance is often used for general purposes where exact measurements are not critical, such as measuring bulk quantities or for rough estimations. However, for more precise measurements, we rely on other balances with higher sensitivities that can detect weight differences at a much finer scale. These balances are calibrated and maintained regularly to ensure accuracy and reliability in our experimental procedure.
Learn more about sensitive balance here: brainly.com/question/4595317
#SPJ11
A balloon is filled up to 2. 25 L with 4. 76 moles of CO2. If we add 8. 74 moles of CO2 to the amount we already had in the balloon, what will the new volume be?
Answers
The new volume of the balloon will be 3. 09 L when we add 8. 74 moles of \(CO_2\) to the initial volume of 2. 25 L.
The new volume of the balloon, we need to use the ideal gas law:
PV = nRT
here P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant (8.314 J/mol·K), and T is the temperature in Kelvin.
We are given that the initial volume of the balloon is 2. 25 L, and that it contains 4. 76 moles of \(CO_2\). To find the pressure of the gas in the balloon, we can use the ideal gas law:
P = nRT/V
here P is the pressure, n is the number of moles of gas, R is the gas constant, T is the temperature in Kelvin, and V is the volume of the balloon. Substituting the given values, we get:
P = (4. 76 moles)(8. 314 J/mol·K)/(2. 25 L)
P = 3. 503 kPa
Now we can use the ideal gas law to find the new volume of the balloon when we add 8. 74 moles of \(CO_2\):
V = PVold + nRT
V = (3. 503 kPa)(2. 25 L) + (8. 74 moles)(8. 314 J/mol·K) x (300 K)
V = 2. 67 L + 271. 94 mol x 8. 314 J/mol·K
V = 3. 09 L
Therefore, the new volume of the balloon will be 3. 09 L when we add 8. 74 moles of \(CO_2\) to the initial volume of 2. 25 L.
Learn more about volume visit: brainly.com/question/29796637
#SPJ4
An inert tracer pulse test produced the following tracer concentrations at the reactor exit. For the first order liquid phase reaction below, carried out at the same conditions as the pulse test (same flow rate, temperature, etc.), use the segregation model to calculate the conversion of A for this isothermal reactor. AB+C ra=-kCA k=0.1 min (at reaction temperature) Cao = 1 mol/L Tracer Time Conc. (min) (M) 0 0.0 1 1.0 2. 2.51 3 5.0 4 2.5 5 1.0 6 0.0
Answers
The segregation model for an inert tracer pulse test can be used to determine the conversion of A for a liquid-phase first-order reaction. The segregation model is a model that is based on the assumption that the tracer concentration in the reactor's fluid stream is proportional to the concentration of A,
and it can be expressed as a function of time.The segregation model's is Ctr = Cao(1- e^(-kt)), where Ctr is the tracer concentration in the reactor's fluid stream, Cao is the initial concentration of the tracer, k is the rate constant of the first-order reaction, and t is time.The concentration of A at the reactor's exit can be calculated by dividing the tracer concentration at each time interval by the segregation model's as shown below.Explanation:Ctr = Cao(1- e^(-kt))Segregation model's= Ctr / Cao,
the concentration of A at reactor exit = Ctr / (k * V)We can compute the value of k * V by dividing the area under the curve by Cao. Using the trapezoidal rule, we can determine the area under the curve. Time (min)Tracer Conc. (M) 0 0.0 1 1.0 2 2.51 3 5.0 4 2.5 5 1.0 6 0.0Area under the curve = (1/2)(1.0 + 2.51)(1-0) + (1/2)(2.51 + 5.0)(3-1) + (1/2)(5.0 + 2.5)(4-3) + (1/2)(2.5 + 1.0)(5-4) = 15.265 M * min.The value of k * V = Area under the curve / Cao = 15.265 / 1 = 15.265 min^-1. The conversion of A can be calculated by substituting the above values into the segregation model's main answer.Ctr = Cao(1- e^(-kt))where t = 6, k = 15.265, and Cao = 1 mol/L.Ctr = 1(1- e^(-(15.265)(6))) = 0.993 mol/LThe concentration of A at the reactor's exit is Ctr / (k * V) = 0.993 / (15.265 * 1) = 0.065 or 6.5%.Therefore, the conversion of A for this isothermal reactor is 6.5%.
TO know more about that segregation visit:
https://brainly.com/question/14330237
#SPJ11
Which statement most accurately describes a chemical equation?
a. in a chemical reaction, the mass of the energy change must be equal to the energy used
b. in a chemical reaction, the mass of the products must equal the mass of the reactants
c. in a chemical reaction, the mass of each product must be the same
d. in a chemical reaction, the combined mass of the container the substances is always equal
Answers
Answer: A
Explanation: