Answers
Explanation:
first you get moles of silver
n=m/M
hence you add no of moles to this equation
c=nv
v=n/c
The volume of 45.6g of silver if the density of silver is 10.5g/mL is 4.342 ml. The correct option is A.
What is Volume?Volume is the space occupied by a three-dimensional object. The volume of any object can be calculated by dividing the mass by its density. It is a scalar quantity. It is the total weight is that object.
Silver is an element in the periodic table. It is non-metal, and it is used in making ornaments and in medicines. The volume of the solver is calculated, and the mass and density are given.
\(\rm{Volume = \dfrac{mass}{density}}\)
The mass of silver is given, 45.6g
The density of the element is 10.5g/mL
Putting the value in the equation
The density and the mass would be divided.
Volume = 45.6g / 10.5 = 4.342
Thus, the volume of silver is 4.342 ml. The correct option is A.
To learn more about Volume, refer to the link:
https://brainly.com/question/2472349
#SPJ2
Related Questions
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
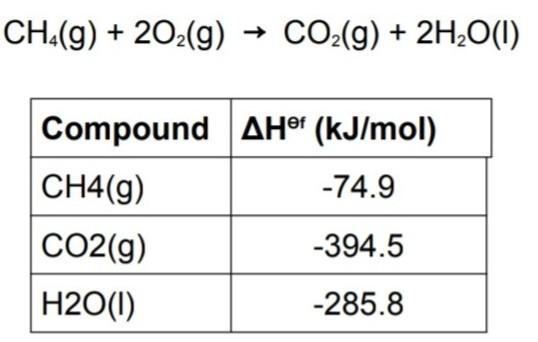
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
A solution containing 113. g of KCl in 270. g of H2O at 50 ∘C is cooled to 20 ∘C . It goes to 34 degrees Celsius when cooled down.
Answers
The solution of KCl in water will undergo precipitation of KCl crystals as the temperature decreases from 50 °C to 20 °C and then to 34 °C due to the decreasing solubility of KCl in water.
What happens to a solution of KCl in water when cooled from 50 °C to 20 °C and then to 34 °C?
When the solution of KCl in water is cooled from 50 °C to 20 °C, the solubility of KCl in water decreases. As a result, some of the KCl will start to come out of the solution and form solid crystals.
Then, when the temperature of the solution is further decreased from 20 °C to 34 °C, the solubility of KCl in water decreases even further, causing more KCl to come out of the solution and form solid crystals. This is known as precipitation.
The amount of KCl that will come out of the solution depends on the solubility of KCl at the respective temperatures. The solubility of KCl in water is about 34 g/100 mL at 20 °C and about 42 g/100 mL at 34 °C. Therefore, when the solution is cooled from 50 °C to 20 °C, some KCl will come out of solution until the concentration in solution reaches about 34 g/100 mL. Further cooling to 34 °C will cause more KCl to come out of solution until the concentration in solution reaches about 42 g/100 mL.
The exact amount of KCl that will come out of solution can be calculated using thermodynamic models and experimental data on the solubility of KCl in water at different temperatures.
To learn more about precipitation, visit: https://brainly.com/question/14160641
#SPJ1
how does the change of macroeconomic equilibrium relate to the 'goals of policy makers?
Answers
Answer:
In thinking about the overall health of the macroeconomy, it is useful to consider three primary goals: economic growth, full employment (or low unemployment), and stable prices (or low inflation).
What are the large plates that move? O gigantic plates O tectonic plates O volcanic plates O rock plates
Answers
Answer:
Tectonic plates
Explanation:
Tectonic plates are large plates that moves. There are different types of plate motion as a result of mantle convection.
A tectonic plate is better as referred to as the lithospheric plate. It is made up of a part of the upper mantle and the overlying crust. Together, they move over the fluid asthenosphere below. The mantle below drives the lithosphere above.Identify each of the following as endothermic or exothermic.
a. Water in a pond evaporates.
b. Methane gas burns on a stove top.
c. Water freezes to form ice.
d. Energy flows from the system to the surroundings.
e. Energy flows from the surroundings to the system.
Answers
Answer:
Identify each of the following as endothermic or exothermic.
a. Water in a pond evaporates.
b. Methane gas burns on a stovetop.
c. Water freezes to form ice.
d. Energy flows from the system to the surroundings.
e. Energy flows from the surroundings to the system.
Explanation:
An exothermic reaction is the one in which heat energy is released.
An endothermic reaction is one in which heat energy is absorbed.
a. Water in a pond evaporates.
This process absorbs heat energy.
Hence, this is an example of an endothermic process.
b. Methane gas burns on a stovetop and release heat energy and hence this is an example of an exothermic reaction.
c. Water freezes to form ice.
In this process heat energy is released.So this is an example of exothermic reaction.
d. Energy flows from the system to the surroundings.
That means heat energy is released into the surroundings.
So, this is an example of exothermic process.
e. Energy flows from the surroundings to the system.
That means energy is absorbed by the system.
So, it is an endothermic process.
lonic bonds form between oppositely charged
Answers
answer:
ionic bonds form between oppositely charged ions
explanation:
an ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. a covalent bond involves a pair of electrons being shared between atoms.What is the change of an atom of one element (atom) to an atom of different element called?
Answers
What is the mass of 20 moles of fluorine?
Answers
Answer:
380g
Explanation:
Given parameters:
Number of moles of Fluorine = 20moles
Unknown:
Mass of Fluorine = ?
Solution:
The number of moles is the unit of quantifying the amount of particles in a substance.
So;
Mass of a substance = number of moles x molar mass
Molar mass of F = 19g/mol
Now;
Mass of F = 20 x 19 = 380g
Define density. Plz help me
Answers
52.6 g sample of granite initially at 125°C was added to a coffee cup Killorin mentor the calorimeter held 100 mL of water at 20°C what will be the final temperature in the calorimeter
Answers
The final temperature of the granite and the water in the calorimeter is 8.4°.
What is the final temperature?We know that in accordance to the first law of thermodynamics, energy is neither created nor destroyed but it can be converted from one form to another. This implies that the heat that is lost by the granite has to be equal to the heat that is gained by the water in this case.
Knowing that;
H = mcdT
H = heat lost or gained
c = specific heat capacity of the substance
dT = temperature change
Heat lost by the granite = Heat gained by water
52.6 * 0.79 * (θ - 125) = 100 * 4.18 * (θ - 20)
41.6θ - 5200 = 418θ - 8360
Collecting the like terms
41.6θ - 418θ = - 8360 + 5200
-376.4θ = -3160
θ = -3160/-376.4
θ = 8.4°
Learn more about calorimeter:https://brainly.com/question/4802333
#SPJ1
At this location, it is summer in the Northern Hemisphere because the North Pole is tilted
Away from the sun
Towards the sun

Answers
Answer:
This happens twice a year during Earth's orbit. Near June 21 the north pole is tilted 23.5 degrees toward our Sun and the northern hemisphere experiences summer solstice, the longest day of the northern hemisphere year.
...
Do other planets have seasons?
Uranus
30,589
97.8
Spring Equinox* 2050
Summer Solstice*
points toward the sun.
closer the earth is to the sun the more hot it will be the closer it is to summer,
you can see at D northern hemisphere is closest to sun and the north pole is pointing toward the sun.
Write a scientific explanation that answers the following question: Is matter conserved during a
chemical reaction in both closed and open systems?
Claim:
Evidence:
Reasoning:

Answers
Answer:
Matter is conserved during all chemical reactions because matter cannot be created or destroyed.
Explanation:
Please help!
Hydrochloric acid is a strong acid whereas acetic acid is a weak acid.
i. How would the pH of a 0.01M acetic acid compare to pH value for 0.01M HCl?
(Explain in your own words without calculating)
ii. Calculate the pH of a 0.01 M acetic acid.

Answers
Because HCl is a stronger acid than acetic acid, the pH of 0.01M acetic acid has greater value than the pH of 0.01M HCl. 2.88 is the pH of a 0.01 M acetic acid.
What is acid?Any hydrogen that comprises a material capable of giving a proton (a hydrogen ion) to another chemical is defined as acid. A base is indeed a molecule or ion that can receive a hydronium ion from just an acid.
1)Because HCl is a stronger acid than acetic acid, the pH of 0.01M acetic acid has greater value than the pH of 0.01M HCl. The pH value of stronger acid is lower.
2)CH\(_3\)COOH + H\(_2\)O ⇄ CH\(_3\)COO⁻+ H\(_3\)O⁺
0.01 0 0
-x +x +x
0.01-x +x +x
Ka=[ CH\(_3\)COO⁻][H\(_3\)O⁺]/[CH\(_3\)COOH]
1.8×10⁻⁵ = [x][x ]/[ 0.01-x ]
x=1.34×10⁻³
pH = -log[H⁺]
= -log[1.34×10⁻³]
=2.88
Therefore, because HCl is a stronger acid than acetic acid, the pH of 0.01M acetic acid has greater value than the pH of 0.01M HCl. 2.88 is the pH of a 0.01 M acetic acid.
To learn more about acid, here:
https://brainly.com/question/29775793
#SPJ9
(Acellus) Oxygen can form a maximum of ____ bonds.
A. 2
B. 3
Answers
Answer:
2
Explanation:
Oxygen has 2 valence electrons.
Therefore, the number of bonds it can form with other atoms is equal to the number of valence electrons.
Hence,
Oxygen can form a maximum of 2 bonds.
Which sample (10.0 g of water or 10.0 g of ethanol) would require more heat to raise the temperature by 10.0°C?
Answers
10.0 g of ethanol would require more heat to raise the temperature by 10.0°C Because it has a higher boiling point.
The boiling point of a liquid is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure of the liquid’s environment. At this temperature, the liquid is converted into a vapour.
The normal boiling point is high for liquids with strong intermolecular attractions and low for liquids with weak intermolecular attractions.
10.0 g of ethanol would require more heat to raise the temperature by 10.0°C because it has a higher boiling point.
Learn more about Boiling point, here:
https://brainly.com/question/2153588
#SPJ1
which element has the electrons configuration 1s22s22p63s23p64s23d104p2
Answers
The element with the electron configuration 1s22s22p63s23p64s23d104p2 is Silicon (Si).
Explanation:
The electron configuration of an element describes the arrangement of electrons in its atoms. The numbers and letters in the configuration represent the energy levels (n), sublevels (s, p, d, f), and the number of electrons in each sublevel.
In this case, the electron configuration of the element is:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Breaking this down, we can see that the element has:
- 2 electrons in the 1s sublevel
- 2 electrons in the 2s sublevel
- 6 electrons in the 2p sublevel
- 2 electrons in the 3s sublevel
- 6 electrons in the 3p sublevel
- 2 electrons in the 4s sublevel
- 10 electrons in the 3d sublevel
- 2 electrons in the 4p sublevel
Based on the number of electrons in the outermost energy level (valence electrons), we can determine that this element is in group 14 of the periodic table. Looking at the periodic table, we can see that the
Which elements occupy the same group in the periodic table
Answers
Answer:
Elements that occupy the same column on the periodic table
This time, include both the coefficient and exponent. Express 0.00212 in scientific notation.
[?] * times 10^[?]
Enter the coefficient in the green box and the exponent in the yellow box.
Coefficient (green) Exponent (yellow)
_______________ _____________ Enter
![This time, include both the coefficient and exponent. Express 0.00212 in scientific notation.[?] * times](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/lnMi2a3nGuhR8UXZWuiEWNQeM7a27uTR.jpeg)
Answers
Answer: 212
Explanation:
Give IUPAC names for the following compounds:

Answers
Answer:
Explanation:
IUPAC nomenclature is based on naming a molecule's longest chain of carbons connected by single bonds, whether in a continuous chain or in a ring. All deviations, either multiple bonds or atoms other than carbon and hydrogen, are indicated by prefixes or suffixes according to a specific set of priorities
Which compound contains ionic bonds?
1.
N20
2.
Na20
3.
СО
4.
CO2
Answers
Please help me with this question please just help me with what is this is or synthesis singlereplacement
combustion
decomposition
double replacement

Answers
Answer:
1) Combustion
2)Synthesis
3)Single Replacement
4)Decomposition
5)Double Replacement
6)Synthesis
7)Double Replacement
8)Single Replacement
9)Combustion
10)Decomposition
Explanation:
Remeber, decomposisition is breaking things apart, synthesis is building them, cobusion will always make water and CO2 and will always burn O2, single replacemnt will have 1 thing replaced (single) and double replacement will have 2 things replaced (double).
Hope this helps!
how do we gain oxygen from trees
Answers
We gain oxygen from trees through the process of photosynthesis. Photosynthesis is the biochemical process in which green plants, including trees, use sunlight, water, and carbon dioxide to produce oxygen and glucose (a form of sugar).
Trees have specialized cells called chloroplasts, which contain a pigment called chlorophyll. Chlorophyll absorbs sunlight energy.
The tree's leaves capture sunlight and use it to convert carbon dioxide (CO2) from the air and water (H2O) from the roots into glucose (C6H12O6) and oxygen (O2).
During photosynthesis, the chlorophyll in the chloroplasts helps to split water molecules into hydrogen ions (H+) and oxygen atoms (O). The oxygen atoms then combine to form O2 molecules.
The oxygen produced during photosynthesis is released into the atmosphere through tiny pores called stomata found on the surface of the tree's leaves. From there, it mixes with the surrounding air and becomes available for us to breathe.
In summary, trees produce oxygen as a byproduct of photosynthesis. They play a crucial role in maintaining the balance of oxygen and carbon dioxide in the atmosphere, providing us with the oxygen we need for respiration.
For more such questions on Photosynthesis visit:
https://brainly.com/question/20861367
#SPJ8
Write the nuclear symbol for the neutral atom shown. 5 protons, 6 neutrons, 5 electrons. nuclear symbol:

Answers
Answer:
B
Explanation:
the element is boron, therefore B is the symbol
B is the nuclear symbol for the neutral atom having 5 protons, 6 neutrons, 5 electrons. The element is boron.
A specific isotope of an element is depicted succinctly by the nuclear symbol, commonly referred to as the nuclear notation or nuclear symbol notation. The element's symbol, atomic number, and mass number are its three component parts. The element's symbol, which is often one or two letters derived from the element's name, stands in for that particular element. For instance, "H" stands for hydrogen, "O" for oxygen, and "Fe" for iron. The atomic number, which represents the quantity of protons in an atom's nucleus, is commonly shown as a subscript on the left side of the symbol. The element is boron.
To know more about nuclear symbol, here:
https://brainly.com/question/1398857
#SPJ6
Consider a disubstituted aromatic compound. The parent name is benzene and there is a chloro and bromo substituent. Disubstituted benzenes can be described using the terms ortho, meta and para, depending on their relative distance from each other. The terms are often just abbreviated as o, m and p. In addition, the IUPAC name can use locant numbers instead of the descriptor. Br
Select the correct names for the structure.
1. 1-bromo-3-chlorobenzene
2. 3-bromo-1-chlorobenzene
3. meta-bromochlorobenzene
4. o-bromochlorobenzene
5. ortho-bromochlorobenzene
6. m-bromochlorobenzene
Answers
Answer:The correct names for the structure are:
--> 1. 1-bromo-3-chlorobenzene.
--> 3. meta-bromochlorobenzene.
--> 6. m-bromochlorobenzene.
Explanation:
Benzene is the simplest member of the aromatic hydrocarbons. It has a ring structure consisting of six carbon and six hydrogen atoms. This equally means that a benzene can have up to six substituents. One of the chemical properties is that benzene and other members of its series undergo substitution reaction whereby one or more of its six hydrogen atoms is replaced by monoatomic reagents.
Disubstituted benzene consists of two substituents which are described based on either numerical locants or specific words for the three possible forms.
The numerical locant method are used the same naming substitutes of other hydrocarbons. From the question, the numerical locant method was derived through using the following steps:
--> the functional group is benzene
--> there are two substituents which includes bromine( written as bromo) and chlorine ( written as chloro)
--> while placing the number, it's done alphabetically ('1-bromo' comes before '3-chloro') in a clockwise manner. This is to give chorine the lowest locant number.
The second naming method for a disubstituted benzene is the the ortho-, meta-, para- (or their singel letter equivalent) nomenclature method. This is only used for benzene structures.
--> ortho or O : this is used when the substituents are close to each other in the benzene ring.
--> meta or (m) : This is used when the substituents are separated by one carbon in the benzene ring.
--> para or (p): This is used when the substituents are across each other in the benzene ring
From the question, the bromine substituent is separated from the chlorine by one carbon atom, therefore it's meta-bromochlorobenzene or m-bromochlorobenzene.
Zoe left her water bottle capped and in her bedroom. She came back some time later to realize that the bottle was “sweating” and left a ring of liquid on her nightstand
Explain thoroughly the science behind why Zoe’s water bottle is sweating
Answers
Answer:
Condensation
Explanation:
Zoe is quite keen to have noticed what we call condensation. Air contains many components, one of those being water vapor. Like how sugar is soluble in water, water can be said to be "soluble" in air. Water will evaporate into the air to a certain extent. The higher the temperature of the air, the more water the air can hold. If the air has more water that it can hold (potentially because of a temperature decrease), the extra water will come out of the air. Zoe's water bottle was cold, and because the air around Zoe's bottle had cooled down, the air can not hold as much water as it could when it was warm, so the air deposited the extra water in the form of liquid water onto the bottle, giving the illusion that her bottle was sweating.
A 36-kg girl walks to the top of stairs that are 2.0-m high. How much gravitational potential energy does the girl gain
Answers
Ep = 36 kg x 9.8 x 2m
Ep = 705.6 J
ipt A wall that was once white is painted black. Which of the following is definitely true of the painted wall? A. Its chemical properties have changed. B. Its physical properties have changed. C. Both its physical properties and chemical properties have changed. D. None of its characteristic properties have changed.
Answers
Answer:
B It's physical properties changed
Explanation:
Because they painted the outside
Hope this helps
3. During an experiment, 98 g of water is used in the Styrofoam cup. The initial temperature
of water was 23.7°C. A 39.9-g piece of metal with initial temperature of 100.3°C (after
removing from the boiling water) is added to the calorimeter. The final temperature of water
was 28.2°C.
A) calculate the specific heat of the metal
B) identify the metal
C) calculate the percent error
Answers
The percent error is 0.22%. This is a very small error, which suggests that our experimental result is quite accurate.
What is Boiling Point?
Boiling point is the temperature at which a liquid changes state from a liquid to a gas or vapor by the process of boiling. At the boiling point, the vapor pressure of the liquid equals the atmospheric pressure, causing bubbles of vapor to form within the liquid and rise to the surface. The boiling point is a physical property of a substance and can vary depending on the pressure and altitude.
A) To calculate the specific heat of the metal, we can use the equation:
q = m * c * ΔT
q_water = m_water * c_water * ΔT_water
= 98 g * 4.184 J/g°C * (28.2°C - 23.7°C)
= 1903.52 J
where c_water is the specific heat of water, which is 4.184 J/g°C.
Next, we need to calculate the heat released by the metal:
q_metal = -q_water
= -1903.52 J
where the negative sign indicates that the heat is released by the metal and absorbed by the water.
Now, we can calculate the specific heat of the metal:
c_metal = q_metal / (m_metal * ΔT_metal)
= -1903.52 J / (39.9 g * (100.3°C - 23.7°C))
= 0.450 J/g°C
Therefore, the specific heat of the metal is 0.450 J/g°C.
B) To identify the metal, we can compare its specific heat to the known values of specific heats for different metals. The specific heat of the metal we calculated is 0.450 J/g°C. Here are some common metals and their specific heats:
Aluminum: 0.900 J/g°C
Copper: 0.385 J/g°C
Iron: 0.449 J/g°C
Lead: 0.128 J/g°C
Silver: 0.235 J/g°C
Zinc: 0.388 J/g°C
From this, we can see that the specific heat of the metal we calculated is closest to that of iron, which suggests that the metal is likely to be iron.
C) To calculate the percent error, we need to compare the experimental specific heat we calculated (0.450 J/g°C) to the accepted value for iron's specific heat (0.449 J/g°C), using the formula:
percent error = |(0.450 J/g°C - 0.449 J/g°C) / 0.449 J/g°C| * 100%
= 0.22%
Learn more about Boiling Point from the given link
https://brainly.com/question/40140
#SPJ1
PLEASE HELP!!
If you triple the amount of a gas in a balloon, what happens
to the volume of the balloon? (Assume that temperature
and pressure remain constant.)
Answers
Answer:
The volume triples
Explanation:
Assuming that the temperature and pressure are constant means the density would be the same
The equation for density is:
Density = mass/volume
Tripling the amount of gas would triple the mass of the balloon, and since we are keeping the same density, the volume also has to triple