What would happen if we didn’t have the Milky Way
Answers
Related Questions
i need help pls!!!!!!!!!!!
Answers
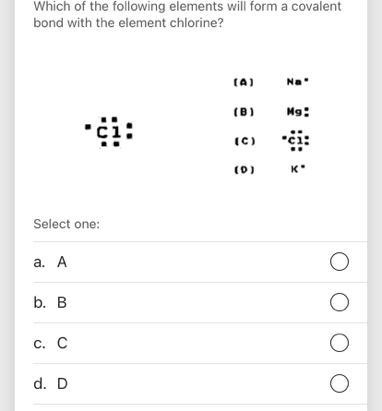
Which element will form covalent bonds with chlorine?
A. Carbon. YES. C is a nonmetal. B. Magnesium. NO. Mg is a metal. C. Potassium. NO. K is a metal. D. Aluminum. NO. Al is a metal.
Chlorine will form covalent bonds with A. Carbon .

Identify the key differences between the Articles of Confederation and the U.S. Constitution. Then explain which document created the better system of government for the new nation, and support your response with the differences you have identified.
22 POINTS + WILL RATE BRAINLEST!!!!!
Answers
The key differences between the Articles of Confederation and the U.S Constitution is the article of confederation is sovereignty in states and the constitution is expand the governments authority.
The document created the better system of government for the new nation is the US constitution.
The Article of confederation , the state have stronger power than the central power and in the US constitution , the power of central government is stronger than the states. The important development was the establishment of three departments. There are three departments of government that is legislative , executive and judicial.
Thus, The key differences between the Articles of Confederation and the U.S. Constitution is the article of confederation is sovereignty in states and the constitution is expand the governments authority.
To learn more about US Constitution here
https://brainly.com/question/29027032
#SPJ1
2. Given the following 2-step mechanism:
Step 1: NO2(g) + Cl2(g) ClNO2(g) + Cl(g)
Step 2: NO2(g) + Cl(g) ClNO2(g)
a. If the second step is the slow step, what is the predicted rate law?
Answers
Answer: Rate = k[NO2][Cl]
Explanation:
The rate law for a reaction describes how the rate of the reaction depends on the concentrations of the reactants. If the second step is the slow step in the mechanism, then the rate law should reflect the rate-determining step, which is the slowest step in the mechanism. In this case, the rate law would be:
Rate = k[NO2][Cl]
This means that the rate of the reaction is directly proportional to the concentration of NO2 and the concentration of Cl.
what is the maximum mass of ethyl alcohol you could boil with 1500 jj of heat, starting from 20 ∘c∘c
Answers
the maximum mass of ethyl alcohol that can be boiled with 1500 J of heat, starting from 20 °C, is approximately 10.49 g.
we know that,
Q = mCΔT
where,Q is the heat energy required to boil the ethyl alcohol,
m is the mass of ethyl alcohol,
C is the specific heat capacity of ethyl alcohol, and
ΔT is the change in temperature required to boil the ethyl alcohol.
We know,
Q = 1500 J (given)
C = 2.44 J/g°C (specific heat capacity of ethyl alcohol)
ΔT = (boiling point of ethyl alcohol) - 20°C (change in temperature required to boil the ethyl alcohol)
The boiling point of ethyl alcohol is 78.37 °C (at standard pressure).
Therefore,
ΔT = 78.37 °C - 20 °C = 58.37 °C
Substituting the values in the formula,Q = mCΔT1500 = m × 2.44 × 58.37m = 1500 / (2.44 × 58.37) ≈ 10.49 g
Therefore, the maximum mass of ethyl alcohol that can be boiled with 1500 J of heat, starting from 20 °C, is approximately 10.49 g.
learn more about heat capacity here
https://brainly.com/question/27991746
#SPJ11
Which of the following is an example of a mixture?
A. CO2 + H2 + O2
B. NaCl
C. C6H12O6
D. Ca
Answers
Explanation:
hey my name is barsha CO2 + H2 + 02
Describe the change that you made that led to the increase in the size of the clawcat population. Explain why the change led to an increase in the clawcat population size.
Answers
The first step is to lessen the number of the organism which consumes clawcats. The second is to enhance the resources Clawncat requires to live.
What is population?Population is the total number of people living in a region (such as a nation or the planet), which is always changing due to births, immigration, and natural mortality.
There are two ways to boost the Clawcat population. The first step is to lessen the number of the organism which consumes clawcats. The second is to enhance the resources Clawncat requires to live, namely by expanding the plantation of Clawncat, providing enough irrigation, giving full access to sunshine, and increasing the richness of the soil through proper and effective fertilization.
Therefore, in the above given ways we can increase the population of Clawncat.
To learn more about population, here:
https://brainly.com/question/27779235
#SPJ1
what is the mass of 7.525 x10^24 particles of sulfur difluoride SF2?
Answers
Answer:
Mass will be equal to 874.56 gram
Explanation:
Molar mass of sulfur = 32 u
Molar mass of Florine = 19 u
Therefore molar mass of \(SF_2\) \(=32+2\times 19=70u\)
So mass of \(6.023\times 10^{23}\) particle is 70 gram
Therefore mass of 1 particle
\(=\frac{70}{6.023\times 10^{23}}=11.62\times 10^{-23}g\)
Therefore mass of \(7.525\times 10^{24}\) particle is
\(7.525\times 10^{24}\times 11.62\times 10^{-23}=874.56gram\)
On a cold, winter day, Sheena rubs her hands together to warm them up. How could Sheena actions be used to explain the Law of Conservation of Energy?
Answers
Answer:
When she rubs her hands together, it causes heat or friction
Keeping in mind the rules for significant digits, what is the product of 0.8954 m and 2.98 m?
Answers
Answer: 3.240576
Explanation:
There are various ways of doing multiplication this is one of them
The wording "keep in mind the rules for significant digits" implies that we are to use these to show where the decimal place goes by approximating the answer. Then conducting the exact calculation.
Consider 0.8 as 8
×
1
10
3
×
8
×
1
10
=
2.4
So the solution is some where a bit above 2.4
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Lets just use the numbers
Multiply both 0.8439 and 3.84 by 1000 to get rid of the decimal place. This makes thing more straight forward. So we end up with:
8439
×
38400
Split up the 38400 into 30000+ 8000 + 400
30000
×
8439
=
253170000
.
8000
×
8439
=
.
67512000
.
.
400
×
8439
=
.
.
3375600
−−−−−−−−−
←
Add
.
324057600
By our approximation we are expecting the form of
2.4
+
small amount
So the answer is
3.240576
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Check by calculator:
0.8439
×
3.84
=
3.240576
Which of the following conditions must be met for a volume ratio to be created from a balanced chemical equation? (3 pm
O Changing temperature and pressure
Constant temperature and pressure
Changing temperature and constant pressure
Constant temperature and changing pressure
Answers
Answer:
The answer will be Constant temperature and pressure
Explanation:
Just took the test and it was correct 100%!!!
Answer:
Constant temperature and pressure
Explanation:
took the test
What is the charge of Mn in MnO4- ?
Answers
Answer: +7
Explanation: Sum of all the oxidation numbers in a polyatomic ion is equal to the charge on the ion. Thus, the total charge on the polyatomic anion is −1. Thus, the oxidation number of Mn in MnO−4 is +7.
1- A ground state atom has the electron configuration
, Kr ] 5s²4d105p² . How many valence electrons does this atom have
? Enter a number .
2- A ground state atom has the electron configuration
. [ A ground state atom has the electron configuration, \( [K r] 5 s^{2} 4 d^{10} 5 p^{2} \). How many valence electrons does this atom have? Enter a number.
A ground state atom has the electron configur
Answers
1.The given electron configuration, [Kr] 5s²4d¹⁰5p², corresponds to the element Selenium (Se) in its ground state has 4 valence shell electrons.
2.The given electron configuration, [Kr] 5s²4d¹⁰5p², corresponds to the element Xenon (Xe) in its ground state has 18 valence electrons.
1.To determine the number of valence electrons, we look at the outermost energy level, which is the 5th energy level (n=5). The 5th energy level consists of the 5s, 5p, and 5d orbitals. In this case, the 5s² and 5p² orbitals are occupied, totaling 4 valence electrons.
2.To determine the number of valence electrons, we look at the outermost energy level, which is the 5th energy level (n=5). The 5th energy level consists of the 5s, 5p, and 5d orbitals. In this case, all the orbitals in the 5th energy level are fully occupied (5s², 5p⁶, 5d¹⁰), totaling 18 valence electrons.
Learn more about valence electrons here:
brainly.com/question/28977387
#SPJ11
One important function of the atmosphere is to provide the Earth and its people with the water needed for life.
T
True
F
False
Answers
Which elements are most vigorous and which elements are least vigorous. (a) sodium and iodine. (b) lithium and iodine. (c) potassium and fluorine. (d) potassium and bromine. (e) sodium and bromine
Answers
The elements that are most vigorous would be (c) potassium and fluorine.
The elements that are the least vigorous would be (d) potassium and bromine
Which elements are reactive ?Potassium is a metal, less reactive than sodium but more reactive than lithium, and fluorine is a highly reactive nonmetal, so both elements would be considered vigorous.
Sodium is a highly reactive metal and bromine is a nonmetal, less reactive than iodine but more reactive than chlorine, so both elements would be considered vigorous but not as much as other options.
Find out more on vigorous elements at https://brainly.com/question/24155109
#SPJ1
In an exothermic the change in enthalpy is usually
Positive, because the potential energy of the reactants is higher than the potential energy of the products
Neutral, because the potential energy of the products and reactants cancel each other out.
Negative, because the potential energy of the reactants is higher than the potential energy of the products
Positive, because the potential energy of the reactants is lower than the potential energy of the products
Answers
Answer:
Negative, because the potential energy of the reactants is higher than the potential energy of the products
Explanation:
Exothermic reaction is a reaction in which heat is released to surrounding. This is due to the fact that the heat content of the reactant is higher than the heat content of product thus producing a negative enthalpy change (ΔH) i.e
Enthalpy change = Heat of product – Heat of reactant
ΔH = Hp – Hr = negative
Considering the options given in the question above, the correct answer is:
Negative, because the potential energy of the reactants is higher than the potential energy of the products
how many grams of c8h18 could be combusted by this quantity of o2 , assuming complete combustion with formation of co2 and h2o ?
Answers
Based on the equation of the reaction;
a. the number of moles of oxygen, n = 0.00374 moles
b. the moles of CₐH₁₈ required = 0.00044 moles
What are the moles of oxygen in the given volume of air?The number of moles of oxygen in the given volume of air is calculated using the ideal gas equation.
The ideal gas equation is given below:
PV = nRT
where;
P is pressureV is volumen is the number of molesR is molar gas constant = 0.082 L.atm/mol/KT is temperatureData given:
P = 0.980 atm; V = 525 cm³ or 0.525 L; T = 71°C or 344 K;
The number of moles of oxygen, n = 0.205 * 0.98 * 0.525 / (0.082 * 344)
The number of moles of oxygen, n = 0.00374 moles
b. The equation of reaction is given below:
2 CₐH₁₈ + 17 O₂ ---> 16 CO₂ + 18 H₂O
Moles of CₐH₁₈ required = 0.00374 * 2/17 moles
Moles of CₐH₁₈ required = 0.00044 moles
Learn more about ideal gas equation at: https://brainly.com/question/25290815
#SPJ1
Complete question:
Assume that a single cylinder of an automobile engine has a volume of 525 cm3 .
part A: If the cylinder is full of air at 71 ∘C and 0.980 atm, how many moles of O2 are present? (The mole fraction of O2 in dry air is 0.2095.)
part B: How many grams of C8H18 could be combusted by this quantity of O2, assuming complete combustion with formation of CO2 and H2O? i got the answer for part A, i just cant seem to get part B correct
What effect would increasing the amount of lauric acid present in the test tube have on the freezing point and the shape/ features of the graph?
(This is a freezing point of lauric acid lab question require at least five sentences)
Answers
Lauric acid, 300 g
Benzoic acid, 30 g
Paper towels
Advance Preparation
It is important that you do the laboratory yourself beforehand. Time may be saved by pre-filling the test-tubes with about 25 g of lauric acid and having the hot and warm water baths ready. [NOTE: The mass of the lauric acid in the test-tubes must be determined to the nearest gram.]
what does le chateliter's principle state
Answers
what is the mass number notation for silicon ?
Answers
Answer: atomic number 14 and atomic mass 20.086
Explanation:
Have a graph
As the atomic number increases in Group 17, there is an increase in -
Answers
Answer:The number of valence electrons in an atom increases down the group due to the increase in energy levels at progressively lower levels. The electrons are progressively further from the nucleus; therefore, the nucleus and the electrons are not as attracted to each other. An increase in shielding is observed.
Explanation: Read this and you'll find your answer~! I hope i helped you~! Have an GREAT day~! <\3
Given the following equation, cu +2 agno3 -> cu(no3)2 + 2ag if 89.5 g of silver is produced, how many grams of cu reacted? conversion factors: 1 mole ag=107.87 g ag 1 mole cu= 2 mole ag 1 mole cu = 63.55g cu
Answers
If 89.5 grams of silver is produced, 105.45 grams of Cu reacted.
The Chemical equation is
Cu + 2AgNO₃ → Cu(NO₃)₂ + 2Ag
The equation is balanced,
89.5 grams of Silver is produced in the reaction.
It is asked that how much Cu will be produced.
The amount of Copper can be calculated by using mole concept,
From the reaction,
When 1 moles of Copper Reacts, 2 moles of Ag are formed.
So, we can say,
Mole of Cu (n) = 2 x Moles of Ag
Mole of a compound can be found by using the formula,
Moles = Given mass of compound/Molar mass of the compound
So,
n = 2 x 89.5/107.87 moles
n = 2 x 0.82 moles
n = 1.64 moles.
Moles of Copper are 1.64.
So, the mass of Cu reacted will be
1 mole Cu = 63.55 grams
1.64 moles Cu = 1.64 x 63.55 grams
1.64 moles of Cu = 105.45 grams.
To produce 89.5 grams of silver, 105.45 grams of Copper would have reacted.
To know more about Mole Concept, visit,
https://brainly.com/question/16488605
#SPJ9
Which is the goal of technology?
to expand comprehension
to make life easier
to apply knowledge
to improve communication
Answers
Answer:
B. To make life easier.
Explanation:
Although all of the above makes sense, the answer should be B. because it is to help improve how we live.
I hope it helps! Have a great day!
Muffin~
The goal of technology is to make life easier.
What is goal?A goal is "an idea of the future or desired result that a person or a group of people envision, plan and commit to achieve".
What is technology?
Technology is "the use of scientific knowledge for practical purposes or applications, whether in industry or in our everyday lives".
Hence, the goal of technology is to make our life better.
To learn more about technology here
https://brainly.com/question/27354061
#SPJ2
Fill in the blank by selecting the correct coefficient that goes in front of the molecule H2

Answers
Answer:
2
Explanation:
Because 2 oxygen atoms in reactant are balanced only when 2H2O is produced.
Propose a structure for compounds consistent with the following mass spectral data: (a) A ketone with M+=86 and fragments at m/z=71 and m/z=43 (b) An alcohol withi M+=88 and fragments at m/z=73, m/z=70, and m/z=59 (c) A hydrocarbon with M+=84
Answers
The ketone that can be shown by the description have been shown in the image attached.
What does mass spectroscopy show us?A strong analytical method known as mass spectrometry can reveal important details about the make-up, structure, and characteristics of molecules. It enables researchers to pinpoint and examine a sample's chemical and isotopic features.
The mass of a molecule or an ion can be precisely determined using mass spectrometry. The mass-to-charge ratio (m/z) measurement enables the recognition and verification of a compound's molecular formula. Particularly relevant to the study of pharmaceutical analysis, biochemistry, and organic chemistry.
Learn more about spectroscopy:https://brainly.com/question/31594990
#SPJ4

he first three ionization energies through of a period element have the following pattern: make a reasonable guess about which element this is. enter its chemical symbol below.
Answers
On the basis of the pattern of the ionization energy through a period shown by the element, the element would be aluminium and it has the chemical symbol Al.
Ionization energy is the energy required to extract one electron from the outermost shell of the element.
Electron is extracted from the outermost shell it is called ionization enthalpy 1, when we remove the second electron from the outermost shell it is called ionization enthalpy 2 and so on.
From the given pattern, we can see that the the ionization enthalpy is increasing drastically with every successful elimination of electron.
If the pattern is of the same element, then this element has to be aluminum most probably.
The chemical symbol for aluminum is Al.
To know more about ionization energy, visit,
https://brainly.com/question/20658080
#SPJ4
number the steps for balancing equations:
Use coefficients to increase the atoms on each side.
Check to make sure you have the same number of each type of atom on each side.
Count the atoms on each side.
ldentify the atoms on each side.
Answers
Answer:
identify the atoms on each side.
Count the atoms on each side.
Use coefficients to increase the atoms on each side.
Check to make sure you have the same number of each type of atom on each side.
Explanation:
The concept behind balancing chemical equations is to ensure that they comply with the law of conservation of matter. This helps to make chemical equations quantitatively meaningful.
First, identify the atoms on each side of the expression. Then count these atoms. Assign appropriate numeric coefficient to the species. Then check to make sure there are equal number of each type of atoms on each side.The subscript of the formula must not be changed in an attempt to balance a chemical equation.
Which of the following functional groups CANNOT hydrogen bond with itself? (select all) 1) Ethers 2) Tertiary amines 3) Esters 4) Carboxylic acids
Answers
Among the given options, the functional group that cannot hydrogen bond with itself is: 1) Ethers Ethers, which have the general formula R-O-R', consist of two alkyl or aryl groups bonded to an oxygen atom.
While oxygen is capable of forming hydrogen bonds with hydrogen atoms from other functional groups, ethers themselves do not have hydrogen atoms directly bonded to the oxygen atom. As a result, ethers lack the necessary hydrogen bonding donor or acceptor sites required for intermolecular hydrogen bonding.
2) Tertiary amines: Although they lack a hydrogen atom directly bonded to the nitrogen atom, they can still participate in hydrogen bonding as hydrogen bond acceptors.
3) Esters: The oxygen atom in the ester functional group can act as both a hydrogen bond donor and acceptor, enabling intermolecular hydrogen bonding.
4) Carboxylic acids: Carboxylic acids have a hydrogen atom bonded to the oxygen of the carboxyl group, making them capable of forming hydrogen bonds with other carboxylic acid molecules through the oxygen and hydrogen atoms.
Learn more about functional group here: brainly.com/question/31328777
#SPJ11
Avogadro's number is the number of particles in one gram of a carbon-12 atom. true false
Answers
False.
12 grams of carbon-12 constitutes 1 mole of carbon. 1 mole of any substance contains Avogadro number of particles.
A mole is a unit of measurement in chemistry.
one mole of something is equal to as many of that something as there are atoms in 12 grams of the isotope carbon-12.
Avogadro’s wide variety, the wide variety of gadgets in a single mole of any substance (described as its molecular weight in grams), is the same as 6.02214076 × 1023. The gadgets can be electrons, atoms, ions, or molecules, relying on the character of the substance and the individual of the reaction.
To learn more about carbon-12 atoms, please refer to the link below:
https://brainly.com/question/1548721
Answer: False
Explanation: Did the exam
a glass vessel fitted with a stopcock has a mass of 337.428g when evacuated completely. when filled with ar, it has a mass of 339.854g. when evacuated and refilled with a mixture of ne and ar (under the same conditions of temp and pressure), it has a mass of 339.076g. calculate the mole fraction of ne in the mixture
Answers
a glass vessel fitted with a stopcock has a mass of 337.428g when evacuated completely. when filled with ar, it has a mass of 339.854g. when evacuated and refilled with a mixture of ne and ar. the mole fraction of ne in the mixture is 64,8%
You filled the first run with m = 339.854g - 337.428g = 2.426g of pure Argon. which is equivalent to N = m / M(Ar) = 2.426g / 39.948g/mol = 0.06073 mol. Assume the gas in the vessel behaves perfectly. The amount in the vessel is then given by: N = pV / (RT) and is independent of the type of gas. If you fill the vessel with the same mixture of N(Ne) moles of Neon and N(Ar) moles of Argon as in the first run, the total number of moles will be the same: N = 0.06073 mol N(Ne) + N(Ar) = N. The total mass of gas is calculated as: N(Ne) M(Ne) + N(Ar) M(Ar) = m', which is measured as: m' = 339.07g - 337.428g = 1.648g. Next, initiate the mole fraction of Neon x(Ne) = N(Ne)/N from the simple equation for the number of moles: N(Ar)/N = 1 - x(Ar) (Ne). Divide the mass equation by N to get: x(Ne) M(Ne) + (1-x(Ne)) M(Ar) = m'/N. As a result, the mole fraction of Neon is calculated as follows: x(Ne) = (M(Ar) - m'/N) = (M(Ar) - M(Ne)) = (39.948g/mol - 1.648g/0.0607mol) / (39.948g/mol - 20.1797g/mol) = 64,8%.
Learn more about mole fraction here:
https://brainly.com/question/29905133
#SPJ4
The area of an object is calculated from experimental data to be 24.6623 cm2. The ± absolute error in the area was determined to be ± 0.6 cm2. The area should be reported, in cm 2 , as A. 25 B. 24.7 C. 24.66 D. 24.6623 E. 24.662
Answers
we should take out from point