Would steak be considered abiotic or biotic and why?
Answers
Answer:
Steak is abotic living organisms are abotic and steak was abiotic
Explanation:
Answer:
Steak would be considered biotic
Explanation:
why? because biotic can mean things which are derived from living organisms. Steak comes form cows, and cows and living organisms.
Related Questions
What are some possible factors that must remain constant during the testing
Answers
Answer:
Four basic components that affect the validity of an experiment are the control, independent and dependent variables, and constants. These basic requirements need to be present and identified to consider an experiment valid.
What is the difference between mineral and non-mineral nutrients? provide an example of each.
Answers
Many essential elements are needed for the growth and developments of plants. The mineral nutrients are essential for all organisms. Calcium, potassium, etc. are mineral nutrients whereas hydrogen, oxygen, etc. are non mineral nutrients.
What are mineral nutrients?The mineral nutrients are defined as the inorganic substances that must be ingested and absorbed in required amounts to perform several metabolic or structural functions in the body.
The non-mineral nutrients are defined as the elements which are synthesized by the body. They are required only in trace amounts. The important non-mineral nutrients include oxygen, hydrogen and carbon.
The mineral nutrients are the macronutrients which include calcium, potassium, phosphorous, etc. The plants get all the required mineral nutrients from the soil. The mineral nutrients being elements, cannot be synthesized biochemically by the living organisms.
All living organisms get minerals from plants or animals.
To know more about mineral nutrients, visit;
https://brainly.com/question/24330470
#SPJ2
balance the equation:2Na+3H2O-2NaOH+H2
Answers
Answer:
2Na + 2H2O → 2NaOH + H2
Explanation:
A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the products.
2.
Copy and complete the following table by giving the formulae of the
compounds formed:
Na+
Ca²+
K+
Mg2+
A1³+
NH4+
OH-
NO3™ SO4²- CO3²-
MnO4 PO4³-
Answers
The formulae of the chemical compounds formed are as follows:
Na⁺ : NaClCa²⁺ : CaSO₄K⁺ : K₂SO₄Mg²⁺ : MgCl₂Al³⁺ : Al(SO₄)₃NH₄⁺ : (NH₄)₂SO₄OH⁻ : NaOHNO₃⁻ : NaNO₃SO₄²⁻ : Na₂SO₄CO₃²⁻ : Na₂CO₃MnO₄⁻ : KMnO₄PO₄³⁻ Ca₃(PO₄)₂What are chemical compounds?A chemical compound is formed when two or more elements are combined together in a definite proportion. Chemical bonds are formed when the elements interact with one another. These bonds develop as a result of atoms sharing electrons.
Examples of chemical compounds include baking soda, water, and table salt.
Learn more about chemical compounds at: https://brainly.com/question/29030999
#SPJ1
Plz help me i have to give this by today pls...........
.Write down the reaction with steam ,and write down the observation
Answers
3. Two boxes need to be moved. Box A is 10 kg. Box B is 3 kg. Which box will 1 point
require more force to move?
BOXA
BOX
Answers
Answer:
box A
Explanation:
10 is bigger than 3 since 10 is bigger it has more force in it
what will be the ph of a buffer solution with an acid (pka2.9) whose concentration is exactly 10.% that of its conjugate base?provide your answer below:
Answers
The ph of a buffer solution with an acid (pka2.9) whose concentration is exactly 10.% that of its conjugate base is 2.9.
What is buffer solution?A buffer solution is a solution composed of a weak acid and its conjugate base, or vice versa. This type of solution is resistant to changes in pH when small amounts of acid or base are added, making it useful for maintaining a constant environment. Buffers are used in many different applications, including in biochemistry and industrial processes for stabilizing pH, in medical laboratories for blood tests, and in many other industries.
The pH of a buffer solution with an acid (pKa2.9) whose concentration is exactly 10% that of its conjugate base can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([conjugate base]/[acid])
In this case, the concentrations of the acid and its conjugate base are equal, so the equation simplifies to: pH = pKa2.9
Therefore, the pH of this buffer solution is 2.9.
To learn more about buffer solution
https://brainly.com/question/8676275
#SPJ4
The amount of energy needed to apply a force of 1 newton over a distance of 1 meter is also called a.
Answers
Answer:
Joule
Explanation:
This is the answer
Short Essay!
Explain how scientists have used the concept of metallic bonding to account for many of the physical properties of metalssuch as electrical conductivity and malleability .
PLS HELP!!
Answers
Answer:
Explanation:
Because electrons are delocalized around positively charged nuclei, metallic bonding explains many properties of metals. Electrical conductivity: Most metals are excellent electrical conductors because the electrons in the electron sea are free to move and carry charge.
which sequence represents a correct order of historical developments leading to the modern model of the atom
Answers
The modern model of the atom are Discovery of the electron, Rutherford's atomic model, Bohr's atomic model, and the development of quantum mechanics.
What is the modern model of the atom? The modern model of the atom consists of three main components; a nucleus, which contains protons and neutrons, and a cloud of electrons that orbit the nucleus. The protons and neutrons in the nucleus are held together by a strong nuclear force. The electrons are held in orbit around the nucleus by the electromagnetic force. The number of protons in the nucleus determines the type of atom. For example, a hydrogen atom has one proton, while a carbon atom has six protons. The number of neutrons can vary, but the total number of protons and neutrons must equal the atomic mass. The electrons are arranged in shells around the nucleus, with each shell having a specific number of electrons. The number of shells and the arrangement of electrons determines the chemical properties of the atom. The modern model of the atom allows for a better understanding of how atoms interact with each other to form molecules, which are the basis of all matter.To learn more about model of atom refer to:
https://brainly.com/question/4138548
#SPJ1
carbonate buffers are important in regulating the ph of blood at 7.40. if the carbonic acid concentration in a sample of blood is 0.0013 m, determine the bicarbonate ion concentration required to buffer the ph of blood at ph
Answers
The blood buffer has a pH of 7.4, whereas the blood sample has a carbonic acid content of 0.0013 M.
The chemical compound of hydrogen, carbon, and oxygen is known as carbonic acid (H2CO3).It is produced in minute quantities when water dissolves its anhydride, carbon dioxide (CO2). Two covalent double bonds bind the core carbon atom of carbon dioxide, also referred to as carbonic acid gas, to the two oxygen atoms.
Calculation:Calculating the concentration is as follows:pH = pKₐ + log HCO⁻₃/ H₂CO₃
7.4 = - log(4.3 × 10⁻₇)+ log HCO⁻₃/ 0.0013m
log HCO⁻₃/ 0.0013m = 7.4 - 6.37
log HCO⁻₃/ 0.0013m = 1.03
log HCO⁻₃/ 0.0013m = 10¹°⁰³
HCO⁻₃ = 1.3 ₓ 10⁻².
To know more about carbonic acid visit:-
https://brainly.com/question/28175742
#SPJ4
6. In which of the following will the bulb glow?
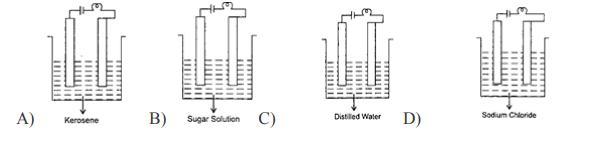
Answers
Answer:
Kerosene
Explanation:
You use process of elimination in this question
None of them except for Kerosene can power a bulb
Explanation:
sodium chloride
thank me later
How are the products represented in the chemical equation for photosynthesis? o2 6co2 c6h12o6 6h2o 6co2 6h2o c6h12o6 6o2
Answers
The chemical equation for photosynthesis is 6CO2+6H2O→C6H12O6+6O2. 6CO2+6H2O→C6H12O6+6O2.
In plants, the process of photosynthesis takes place in the mesophyll of the leaves, inside the chloroplasts.
Photosynthesis is the technique with the aid of which inexperienced plant life and positive other organisms remodel light electricity into chemical strength.
During photosynthesis in green flowers, mild strength is captured and used to convert water, carbon dioxide, and minerals into oxygen and energy-wealthy organic compounds.
Learn more about photosynthesis here: https://brainly.com/question/3529377
#SPJ4
Answer:
The answer is actually:
D
Explanation:

a combustion reaction occurs in a sealed container of constant volume. at the start of the reaction, where only reactants are present, the pressure in the container is 10.0 atm at 400 k. after the combustion reaction is over, all reactants have been consumed and only products are present in the container. at the end of the reaction, the container is returned to 400 k and the pressure is found to be 11.7 atm. which combustion reaction is taking place in the container?
Answers
Based on the change in pressure, we can conclude that the combustion reaction taking place in the container is likely to involve the production of gases that increase the pressure in the container by 1.7 atm.
What are Combustion Reactions?Based on the given information, we can use the ideal gas law to determine the combustion reaction taking place in the container. The ideal gas law is given by the equation:
PV = nRT
where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is temperature.
Since the container is sealed and the volume is constant, we can assume that the number of moles of gas (n) and the volume (V) remain constant throughout the combustion reaction. Therefore, we can compare the initial and final pressures (P1 and P2) at the same temperature (T) to determine the change in pressure (ΔP) caused by the combustion reaction.
Initial pressure (P1) = 10.0 atm
Final pressure (P2) = 11.7 atm
Temperature (T) = 400 K
ΔP = Final Pressure - Initial Pressure
ΔP = 11.7 atm - 10.0 atm
ΔP = 1.7 atm
Since the pressure increased by 1.7 atm and the container has a constant volume, this indicates that there is an increase in the number of moles of gas in the reaction. Without specific reactant information, it is not possible to determine the exact combustion reaction taking place in the container.
To know more about Combustion Reactions:
https://brainly.com/question/30562669
#SPJ11
Galaxies are made of billions of?
Planets
Nebulas
Stars
Meteors
Answers
Hope that helps :D
Complete each nuclear reaction equation.
Superscript 14 subscript 7 upper N plus superscript 4 subscript 2 upper H e right arrow superscript question mark subscript 8 upper O plus superscript 1 subscript question upper H.
Nitrogen transmutes to form an isotope of oxygen. The superscript for oxygen is?
The subscript for hydrogen is?
Superscript 59 subscript question mark upper C o plus superscript 1 subscript 0 n right arrow superscript 60 subscript 27 upper C o.
Cobalt-59 accepts a neutron to form a new isotope. The subscript for cobalt is?
Answers
Nitrogen transmutes to form an isotope of oxygen. The superscript for oxygen is 17
The subscript for hydrogen is 1
Cobalt-59 accepts a neutron to form a new isotope. The subscript for cobalt is 27
Answer:
Oxygen = 17
Hydrogen = 1
Cobalt = 27
Explanation:

HELP PLEASE DUE TODAY

Answers
Answer:
Explanation: The invention of the microscope led to the discovery of the cell by Hooke. While looking at cork, Hooke observed box-shaped structures, which he called “cells” as they reminded him of the cells, or rooms, in monasteries. This discovery led to the development of the classical cell theory.
Answer:
Microscopes help see cells up close and if you have for example a Disease they and see what it looks like up close on you cells and discover what it is
6 spills and 100 squares
If each spill represents one year, what is the half-life of the square?
Answers
Answer:
hi
Explanation:
What is a saturated solution
A. A solution containing more solute than should be possible
B. A solution that cannot dissolve any more solute
C. A solution that is not able to dissolve any of the solute
D. A solution that can still dissolve more solute
Answers
because:
A saturated solution is a solution in which there is so much solute that if there was any more, it would not dissolve.
why is the water a liquid and h2s a gas ?
Answers
Explanation:
This is because the hydrogen bonding in water H2O is stronger than that is hydrogen sulfide H2S.
Be sure to answer all parts. Give the individual reaction orders for all substances and the overall reaction order from the followin rate law: Rate = k[NO2]^2[Cl2] Order with respect to NO2: ___
Order with respect to Cl2: ____
Order overall: ____
Answers
The individual reaction orders for the rate law, Rate = k[NO2]^2[Cl2], are:
Order with respect to NO2: 2
Order with respect to Cl2: 1
To determine the overall reaction order, we add the individual reaction orders:
Order overall: 2 + 1 = 3
Therefore, the overall reaction order is 3.
The reaction order with respect to a particular reactant is the exponent to which its concentration is raised in the rate law. In this case, the rate of the reaction depends on the square of the concentration of NO2 and the concentration of Cl2 raised to the first power.
For more similar questions on concentration:
brainly.com/question/4184101
#SPJ11
How many milliliters of a 0.1000 M NaOH solution would be needed to neutralize a 1.0000-g sample of potassium hydrogen phthalate (KHC8H4O4) to a phenolphthalein endpoint, according to the following balanced equation?
KHC8H4O4(s)+NaOH(aq)------> KNaC8H4O4(aq)+H2O(l)
Answers
Answer: 2) 2HCl(sq) + CaCO3(s) CaCl2(sq) + CO2(g) + H2O (l) No of moles of CaCO3 = amount of the CaCO3 (g)/mw of CaCO3 (g/mole)= 0.8085 g/100 g/mole = 0.008085
Explanation:
How many bromide ions are there in 2.00g of MgBr2?
Answers
There are 1.31 x 1022 bromide ions in 2.00 g of \(MgBr_2\).
The chemical formula of magnesium bromide (\(MgBr_2\)) contains one magnesium ion (\(Mg2^+\)) and two bromide ions (Br-). To find the number of bromide ions in 2.00 g of \(MgBr_2\), we need to use the molar mass of \(MgBr_2\) to determine the number of moles of \(MgBr_2\) present in 2.00 g of the compound, then use the stoichiometry of the chemical formula to determine the number of bromide ions present. First, we need to calculate the molar mass of \(MgBr_2\). The molar mass of \(MgBr_2\) is equal to the sum of the atomic masses of magnesium (Mg) and two bromine (Br) atoms. The atomic mass of Mg is 24.31 g/mol, and the atomic mass of Br is 79.90 g/mol. Molar mass of \(MgBr_2\) = 24.31 g/mol + (2 x 79.90 g/mol) = 184.11 g/mol Next, we can use the molar mass to determine the number of moles of \(MgBr_2\) present in 2.00 g of the compound: Number of moles of \(MgBr_2\) = mass of \(MgBr_2\) / molar mass of \(MgBr_2\)= 2.00 g / 184.11 g/mol
= 0.0109 mol Finally, we can use the stoichiometry of the chemical formula to determine the number of bromide ions present: Number of bromide ions = 2 x number of moles of \(MgBr_2\)
= 2 x 0.0109 mol
= 0.0218 mol Therefore, there are 0.0218 moles of bromide ions in 2.00 g of \(MgBr_2\). To convert this to the number of bromide ions, we can multiply by Avogadro's number (6.02 x 1023): Number of bromide ions = 0.0218 mol x 6.02 x 1023 ions/mol = 1.31 x 1022 ions
For more questions on bromide ions
https://brainly.com/question/29228517
#SPJ8
Acid rain is a form of pollution. acid rain can cause buildings and other structures made of limestone or marble to weaken and break apart. what process does this change represent?
Answers
occurs when acid rain causes buildings and structures made of limestone or marble to weaken and break apart represents a process known as chemical weathering.When these acids come into contact with limestone or marble, a chemical reaction occurs that dissolves the calcium carbonate present in these rocks.
Chemical weathering is the process by which the chemical composition of rocks and minerals is altered due to the interaction with substances such as water, oxygen, or acids. In the case of acid rain, the rainwater combines with pollutants in the air, such as sulfur dioxide and nitrogen oxide, to form sulfuric acid and nitric acid.
This reaction can be summarized as follows Calcium carbonate (present in limestone or marble) reacts with sulfuric acid or nitric acid.. The carbon dioxide gas is released into the atmosphere, while the water-soluble calcium sulfate or calcium nitrate is washed away.
To know more about acid rain visit :-
https://brainly.com/question/53991
#SPJ11
Which of the following can be inferred from the diagram above that shows the dependence of potential Energy on the Internuclear distance between two atoms?A - The atoms form a bond with a bond length of 25 pmB - The atoms form a bond with a bond length of 75 pmс - The net force between the atoms is attractive at 25 pmD - The net force between the atoms is attractive at 75 pm
Answers
The answer is Option B, The atoms form a bond with a bond length of 75 pm.
Explain Potential Energy Surfaces and Inter-nuclear distance.In terms of specific characteristics, typically the locations of the atoms, a potential energy surface (PES) represents the potential energy of a system, particularly a group of atoms. If there is only one coordinate, the surface is known as a potential energy curve or energy profile. The surface may specify the energy as a function of one or more coordinates. It is beneficial to think of a system as having two degrees of freedom, such as two bond lengths, because the value of the energy (analogy: the height of the land) depends on two bond lengths (analogy: the coordinates of the position on the ground).
The Potential Energy Surface illustrates the idea that each atom's and molecule's internal and exterior geometries in a chemical reaction have a distinct potential energy. A smooth energy "landscape" is produced, and chemistry can be examined from a topology perspective as a result (of particles evolving over "valleys""and passes").
Internuclear distance: The bond length is determined by the internuclear distance at which the potential energy minimum occurs. Since the two atoms vibrate approximately this distance due to thermal motion, this is more appropriately known as the equilibrium bond length. In general, the bond length will be shorter the stronger the relationship.
It is occasionally possible to utilize an analytically calculated expression for the energy as a function of the atomic locations for very simple chemical systems or when simplifying approximations are made concerning inter-atomic interactions. Among them is
H + H2 → H2 + H
All atoms are subject to attractive forces, but unless the minimum potential energy is at least of the order of RT, the two atoms cannot endure the disruptive effects of thermal energy for long enough to form a recognizable molecule.
Thus, we can conclude that the two atoms in H2 are connected by a chemical bond. Ar2 cannot exist as a molecule due to the weak attraction between argon atoms, but the van der Walls force is what keeps argon atoms together in liquid and solid states.
To know more about Potential Energy Surfaces and Inter-nuclear distance refer to :
brainly.com/question/18484436
#SPJ4
The graph below shows the changes in the temperature of ice when it is heated from -20 °C to 100 °C.
Heat curve for ice. The graph shows the changes in the temperature of ice when it is heated from negative 20 degrees Celsius to 100 degrees Celsius. The label for the x-axis reads 'Addition of heat over time.' The label for the y-axis reads 'Temperature (degrees Celsius).' There are labels on the graph (from 'A' to 'E') that designate significant events while applying heat to the ice. Portion AB has a slight incline while increasing the heat from negative 20 degrees to 5 degrees; portion BC stays at 5 degrees for a while; portion CD steadily increases in temperature from 5 degrees to 100 degrees over a significant period of time; portion DE stays at 100 degrees for a while.
Based on the graph, which of these conclusions is correct?
Answers
Answer:
D
Explanation:
I did it on a quiz.
In the insoluble and soluble salt lab, the dropper bottles containing the anions to be studied were all choose. Salt solutions. The dropper bottles containing the cations to be studied were all choose. Salt solutions.
Answers
A dropper bottle containing anion to be studied were all phosphate salt solutions. A dropper bottle containing cations to be studied were all iron salt solutions.
A homogenous mixture of two or more components is referred to as a solution. Any phase may have a solution. A solute and a solvent make up a solution. The thing that dissolves in the solvent is called the solute. Solubility is the measure of a solute's ability to dissolve in a solvent. For instance, salt is the solute and water is the solvent in a saline solution.
The chemicals present in lesser concentrations are solutes, whilst the substances present in greater abundance are the solvent in solutions with components in the same phase. In the case of air, the solutes are the gases of oxygen and carbon dioxide, and the solvent is the gas of nitrogen.
To know more about solutions visit : https://brainly.com/question/24058779
#SPJ4
What happens to wavelength as wave frequency increases?
Answers
Answer:
As a wavelength increases in size, its frequency and energy (E) decrease. From these equations you may realize that as the frequency increases, the wavelength gets shorter. As the frequency decreases, the wavelength gets longer.
Explanation:
which of the following statements correctly describe resonance structures? select all that apply.
Molecules that require more than one inwis structure to accurately describe them.
The actual molecule is an average of the Lewis structure.
Moleculos that change back and forth between several different forms Molecules that spin so that double bends paint in ditferent dircctions
Molecules that react with other molecuies and form a hybrid
Answers
The statements correctly describe resonance structures is The actual molecule is an average of the Lewis structure. Therefore, option B is correct.
What is Lewis structure ?The representations known as Lewis structures, often referred to as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures, depict the interactions between the atoms in a molecule as well as any lone pairs of electrons that may be present.
The electronic bonding of a single polyatomic species, including fractional bonds and fractional charges, is described by resonance structures, which are a collection of two or more Lewis structures.
In comparison to separate resonance structures, the resonance hybrid is more stable. The movement of a charge between two or more atoms is frequently represented by resonance structures. These atoms have a more evenly distributed charge, which makes it more stable.
Thus, option B is correct.
To learn more about lewis structure, follow the link;
https://brainly.com/question/4144781
#SPJ1
he scientist had a 35% saline solution that he mixed with 10 milliliters of a 75% saline solution to get a 40% saline solution. How many milliliters of the 35% solution were used? a) 30 milliliters b) 40 milliliters c) 50 milliliters d) 60 milliliters e) 70 milliliters
Answers
The answer is (e) 70 milliliters.
Let's assume that x milliliters of the 35% saline solution were used.
The total amount of saline in the solution can be calculated as follows:
Saline in 35% solution = 0.35 * x
Saline in 75% solution = 0.75 * 10 (since 10 milliliters of the 75% solution were used)
The total amount of saline in the resulting 40% solution can be calculated as:
Saline in 40% solution = 0.40 * (x + 10)
Since the saline is being mixed, the total saline in the resulting solution is equal to the sum of the saline in the individual solutions:
0.35 * x + 0.75 * 10 = 0.40 * (x + 10)
Simplifying the equation:
0.35x + 7.5 = 0.40x + 4
Subtracting 0.35x and 4 from both sides:
7.5 - 4 = 0.40x - 0.35x
3.5 = 0.05x
Dividing both sides by 0.05:
x = 3.5 / 0.05
x = 70
Therefore, 70 milliliters of the 35% saline solution were used. The answer is (e) 70 milliliters.
For more details regarding the saline solution, visit:
https://brainly.com/question/24498665
#SPJ4