4. They are the muscles which have complete control and responsible for all
kinds of body movement
A. Contracting B. Involuntary C. Relaxing D. Voluntary
5. What system that is made up of many bones joined to form a structure?
A. Digestive B. Integumentary C. Muscular
D. Skeletal
6. How do our muscles work?
D. By touching
A. By pulling B. By smelling C. By seeing
7. These are the long bones of our body.
A legs B. Patella
C. Ribs D. Tendons
8. What composes the lower extremities of the body?
A backbone B. Compact bone C. Knee bone D. Pelvic bone
9. An organ that manufactures the blood cells in our body?
A Bone marrow B. Compact bone C. Knee bone D. Pelvic bone
10. Are muscles that move without conscious effort like the beating of our heart.
A contracting B. Involuntary
C. Relaxing
D. Voluntary
Answers
Option B: voluntary muscles are the muscles which have complete control and are responsible for all kinds of movements.
Animals' muscles are the types of tissue that cause movement or motion. Voluntary refers to action taken of one's own free will or will. All of the muscles that are linked to the skeleton are voluntary muscles, hence these muscles are also referred to as skeletal muscles or striated muscles because of the way their muscle fibres give them a striated, or stripy, appearance. This, therefore, tells us that option B is he right choice.
The only muscles that can be actively manipulated are those in the skeleton. Since the muscles are connected to the bones, pulling on them moves the bones. Skeletal muscles are used for every conscious movement a person makes.
To know more about skeletal muscles, refer to the following link:
https://brainly.com/question/12252128
#SPJ4
Related Questions
A sealed flask initially contains pure nitrogen dioxide gas (NO,). Over time, the nitrogen dioxide forms dinitrogen tetroxide gas (N,O.). The graph below shows the relative amounts of (NO,) and (N20.) over time. What is true about the time indicated by the blue arrow?
Answers
This problem is providing information about the equilibrium reaction whereby nitrogen dioxide gas produced dinitrogen tetroxide gas as shown on the attached picture and the following chemical equation:
NO₂ (g) ⇄ N₂O₄ (g)
In such a way, we can consider the given choices to reason the following: the blue arrow is pointing out the arrival to the equilibrium condition for the reaction, a point in which the rate of the forward reaction (formation of N₂O₄) is equal to the rate of the reverse reaction (formation of NO₂), because the molecules will fluctuate to the same relative amounts defined by the equilibrium constant.
Therefore the answer will be the first one on the attached file, which can vary on your online homework format.
It is important to note that the reactant is not used up at any point of the graph (concentration drops to 0) and also, the activation energy cannot be analyzed with this sparse information.
Learn more:
(equilibrium concentrations) https://brainly.com/question/7949757
(this is science not chemistry)

Answers
I'm pretty sure ita A. acceleration
Answer:
it's a friction force
give a reason why it is not advisable to heat magnesium before heating ammonium nitrate
Answers
Answer:
it can result in an increasing risk of the accumulation of decomposition products, self-heating (from the heat released by the slow decomposition reactions)
Magnesium can result in an increased risk of the accumulation of decomposition products, self-heating (from the heat released by the slow decomposition reactions)
What is ammonium nitrate?Ammonium nitrate is used commonly in fertilizers; in pyrotechniques, herbicides, and insecticides; and in the manufacture of nitrous oxide.
When ammonium nitrate is heated, it decomposes exothermically into nitrous oxide and water.
Hence, Magnesium can result in an increased risk of the accumulation of decomposition products, self-heating (from the heat released by the slow decomposition reactions)
Learn more about ammonium nitrate here:
https://brainly.com/question/5148461
#SPJ2
I just want u to check if it’s correct or not no need for explanations
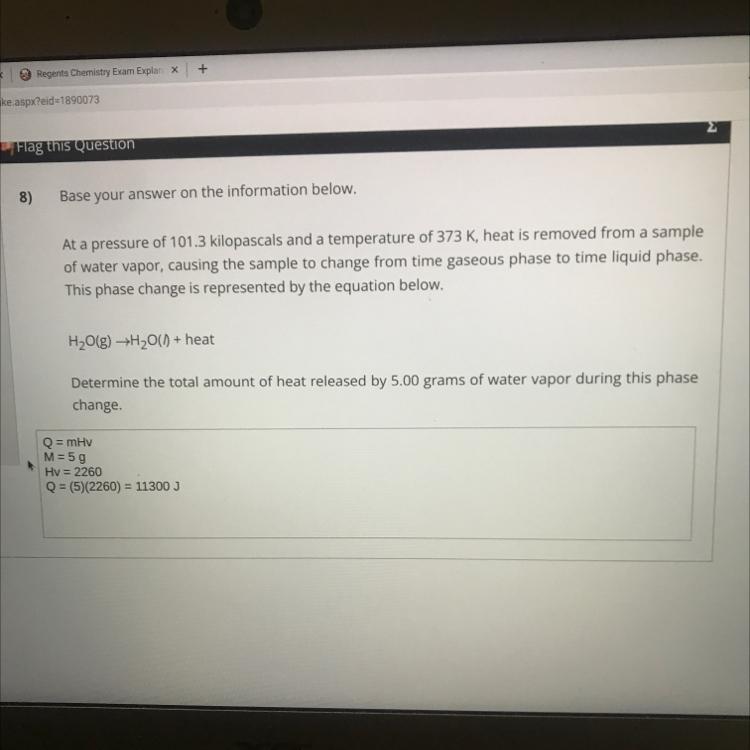
Answers
Given mass = 5g
Heat of vapour = 2260
Heat released during conversion of steam = m * C = 5 * 2260
= 11300J
Your calculations are correct.
The first law of thermodynamics is observed when

Answers
Answer:
Explanation:
The first law of Thermodynamics is known as Conservation because it explains that energy is always maintained within a closed system and cannot be created or destroyed. Therefore, this is observed when there is no longer change in temperature in a system. Mainly because the energy is not being transferred to and from another system. Without this transfer of energy, the energy itself gets conserved within the system and the temperature no longer fluctuates.
6
What is the density of a substance that has a mass of 2.0 g, and when placed in a graduated cylinder
the volume changed from 70 mL to 75 mL?
A 2.5 g/mL
B 7.0 g/mL
C 10. g/mL
D 0.40 g/mL
Answers
The density of the substance having a mass of 2.0 g is 0.4 g/mL (Option D)
How do I determine the density of the substance?First, we shall obtain the volume of the substance. This can be obtained as follow:
Volume of water = 70 mL Volume of water + substance = 75 mL Volume of substance =?Volume of substance = (Volume of water + substance) - (Volume of water)
Volume of substance = 75 - 70
Volume of substance = 5 mL
Finally, we shall determine the density of the substance. This is illustrated below:
Mass of substance = 2.0 gVolume of substance = 5 mLDensity of substance = ?Density = mass / volume
Density of substance = 2 / 5
Density of substance = 0.4 g/mL
Thus, the density is 0.4 g/mL (Option D)
Learn more about density:
https://brainly.com/question/952755
#SPJ1
What do you understand by the terms radial node and nodal plane, as applied to AO wavefunctions? Illustrate your answer using the 2s and 2p AOs. Explain why radial nodes arise from the radial part of the wavefunction, whereas nodal planes arise from the angular part of the wavefunction
Answers
In the context of atomic orbital (AO) wavefunctions, the terms "radial node" and "nodal plane" refer to different aspects of the wavefunction's behavior.
A radial node is a region in the AO wavefunction where the probability of finding an electron is zero along the radial direction. In other words, it represents a spherical shell where the electron is unlikely to be found. The number of radial nodes is determined by the principal quantum number (n) of the orbital. For example, the 2s orbital has one radial node, while the 2p orbital has no radial nodes.
On the other hand, a nodal plane is a flat plane within the AO wavefunction where the probability of finding an electron is zero along a particular direction. It represents a surface that divides the orbital into two regions of opposite phases. The number of nodal planes is determined by the angular quantum numbers (l and m) of the orbital. For example, the 2s orbital has no nodal planes, while the 2p orbital has one nodal plane (the xz or yz plane).
Radial nodes arise from the radial part of the wavefunction because they depend on the distance from the nucleus. The radial part determines the distribution of the electron density as a function of distance, and the nodes correspond to regions where the density drops to zero.
On the other hand, nodal planes arise from the angular part of the wavefunction because they depend on the orientation and shape of the orbital. The angular part describes the angular distribution of the electron density around the nucleus, and the nodal planes correspond to regions where the phase of the wavefunction changes sign.
In summary, radial nodes are related to the distance from the nucleus and arise from the radial part of the wavefunction, while nodal planes are related to the orientation and shape of the orbital and arise from the angular part of the wavefunction. The 2s orbital has one radial node and no nodal planes, while the 2p orbital has no radial nodes and one nodal plane.
learn more about radial node here
https://brainly.com/question/31829965
#SPJ11
IUPAC name for [Fe(NH3)4Cl2]NO3
Answers
Tetraamminedichloridoiron(3) nitrate
Under the Controlled Substance Act (CSA), dispensing a controlled substance (CS) to the ultimate use includes ____________ (Select ALL that Apply)
Answers
Answer:.
pharmacies, prescribers, distributors/wholesalers, researchers, manufacturers, hospitals.
Explanation:
The Controlled Substance Act is a federal drug policy of the United States which regulates the manufacture, use, importation and possession and distribution of certain substances. It was enacted by the United States Congress in the year 1971.
Under the Controlled Substance Act, the dispensing of a controlled substance to the use includes pharmacies, prescribers, distributors/wholesalers, researchers, manufacturers, hospitals, etc.
Name two elements whose outer energy levels are complete with two electrons
Answers
Answer:
ppsuck
Explanation:
f;lhfadlkahglkaga
A gas occupies a volume of 10 liters at a pressure of 0.5 atm. What is the pressure if the volume increases to 25.0 L? *
A.0.4 atm
B.0.3 atm
C.0.2 atm
D.0.1 atm
Answers
Answer:
0.2atm
Explanation:
By Boyle's law
P1V1=P2V2
0.5 x 10 = P2 x 25
therefore, P2= 0.2atm
what is the heat of a reaction, in joules, with a total reaction mixture volume of 55.7 ml if the reaction causes a temperature change of 4.1 oc in a calorimeter?
Answers
The heat of the reaction is determined as 956 J.
What is the heat of the reaction?The heat of the reaction is calculated by applying the following formula as follows;
Q = mcΔθ
where;
m is the mass of the waterc is the specific heat capacity of waterΔθ is the change in temperatureThe mass of the water is obtained from its density;
density of water = 1 g/ml
mass of water = density x volume
mass of water = 1 g/ml x 55.7 ml = 55.7 g
The heat of a reaction is calculated as;
Q = 55.7 x 4.186 x 4.1
Q = 956 J
Learn more about heat of reaction here: https://brainly.com/question/30464598
#SPJ4
30 POINTS
Match the following vocabulary words.
1 .
ellipse
on or about September 22, when the days and nights are equal
2 .
autumnal equinox
an egg-shaped orbit of a heavenly body
3 .
galaxy
one of billions of heavenly systems consisting of numerous stars; an example is the Milky Way
4 .
vernal equinox
the curved pathway of a heavenly body through space
5 .
orbit
the spring equinox, on about March 21, when days and nights are of equal length
Answers
More on space science can be found here:https://brainly.com/question/12842883
#SPJ1
What is the name of C3H8?
Answers
Answer:
Propane
Explanation:
The lowest parts of a transverse wave are called
1.Transversals
2.summits
3.troughs
4.crest
Answers
Choose the one best response for why you discard clean broken glassware, melting point capillaries, pipets or glass test tubes in the broken glass box and not in the regular trash can.
(a) Glassware may still contain chemicals that need to be disposed of properly.
(b) The stockroom cannot charge for broken glassware if it is discarded in the regular trash.
(c) Potential injuries could occur to the janitorial staff if the broken glassware is not seen.
Answers
The one best response for the discard clean broken glassware, melting point capillaries is the option (c) Potential injuries could occur to the janitorial staff if the broken glassware is not seen.
It is very danger to throw out the broken glassware away that has been in the touch with the chemical products in a regular garbage or the recycling containers. This is dangerous because of it can lead to the serious injuries if the worker that collects it from the trash and it is not aware of the presence of the broken glass in the garbage bin.
Thus, Potential injuries can occur to the janitorial staff if the broken glassware is not seen.
To learn more about glassware here
https://brainly.com/question/29414634
#SPJ4
A farmer wants to start growing sweetcorn on his farm. He has found out that sweetcorn grows best in soil with a pH value of approximately 7.5. Explain how he can use the knowledge of acids, alkalis, and neutralisation to find out the pH value of his soil to make sure he gets the best crop possible
Answers
Answer:
The process to use this knowledge is explained as below:
Explanation:
1. Farmer should use an indicator to check the pH value of the soil of the field of the farm.
2. If the field or the farm has alkali soil add acid to reduce the pH value.
3. If the soil of the farm is acidic for the crop add alkali to increase the pH value.
4. It will be a neutralization reaction and changes the pH value of the farm.
5. Weather/leeching into the surrounding soil/plant or animal waste will lead to a change in pH value over time.
6. The pH value will need to be regularly monitored and adjusted.
You are studying a new reaction and measure the ΔH
0
as 1.2 kJ/mol and ΔS
∘
as 24.7 J/mol/K. What is the ΔG
0
for thi s reaction? Report your anser in kJ/mol to the nearest 0.1 kJ/mol. Assume the reaction is occuring at body temperature, 37
∘
C
Answers
The ΔG° for a reaction can be calculated using the equation ΔG° = ΔH° - TΔS°. Given ΔH° = 1.2 kJ/mol, ΔS° = 24.7 J/mol/K, and assuming a temperature of 37°C, we can convert the values and plug them into the equation to determine ΔG° for the reaction.
Convert the temperature from Celsius to Kelvin by adding 273.15:
T = 37°C + 273.15 = 310.15 K.
Next, we convert ΔS° from J/mol/K to kJ/mol/K:
ΔS° = 24.7 J/mol/K / 1000 = 0.0247 kJ/mol/K.
Now we can plug the values into the equation ΔG° = ΔH° - TΔS°, where ΔH° is the enthalpy change, ΔS° is the entropy change, and T is the temperature in Kelvin:
ΔG° = 1.2 kJ/mol - (310.15 K)(0.0247 kJ/mol/K).
Simplifying the equation, we get:
ΔG° ≈ 1.2 kJ/mol - 7.661305 kJ/mol.
Calculating the subtraction, we find:
ΔG° ≈ -6.4613 kJ/mol.
Therefore, the ΔG° for the reaction is approximately -6.5 kJ/mol to the nearest 0.1 kJ/mol.
Learn more about enthalpy here:
https://brainly.com/question/32882904
#SPJ11
Which is a process that involves collecting information and ideas that are supported by belief or opinion?
science
systematics
pseudoscience
hypothesis
Answers
Answer:
The answer is C
Explanation:
I did it on edge
The highest temperature ever recorded in the United States is 134°F at Greenland Ranch, Death Valley, CA, on July 13, 1913.
What is this temperature on the Celsius scale?
What is this temperature on the Kelvin scale?
Answers
The highest temperature ever recorded in the United States, which is 134°F, is equivalent to approximately 56.7°C on the Celsius scale and approximately 329.85 K on the Kelvin scale.
To convert the highest temperature ever recorded in the United States, 134°F at Greenland Ranch, Death Valley, CA, on July 13, 1913, to the Celsius scale, we need to use the formula:
°C = (°F - 32) *\frac{ 5}{9}
Plugging in the values, we get:
°C = (134 - 32) *\frac{ 5}{9} = 56.7°C
To convert this temperature to the Kelvin scale, we need to use the formula:
K = °C + 273.15
Plugging in the value of Celsius we just calculated, we get:
K = 56.7 + 273.15 = 329.85K
Therefore, the highest temperature ever recorded in the United States is equivalent to 56.7°C on the Celsius scale and 329.85K on the Kelvin scale. It is worth noting that the Kelvin scale starts at absolute zero (−273.15°C), making it the preferred temperature scale for scientific calculations.
learn more about Kelvin scale Refer: https://brainly.com/question/28938811
#SPJ11
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
A gas sample with a mass of 0.250g is collected at 150.0°C and 720 mmHg. The volume is 85.0mL. What is the molar mass of the gas?
Answers
The molar mass of the gas, given that 0.250 g of the gas is collected at 150.0 °C and 720 mmHg is 108.7 g/mol
How do i determine the molar mass of the gas?First, we shall obtain the mole of the gas collected. This is shown below:
Volume of gas (V) = 85 mL = 85 / 1000 = 0.085 LTemperature (T) = 150.0 °C = 150 + 273 = 423 KPressure (P) = 720 mmHg = 720 / 760 = 0.947 atmGas constant (R) = 0.0821 atm.L/mol KNumber of mole (n) =?PV = nRT
0.947 × 0.085 = n × 0.0821 × 423
Divide both sides by (0.0821 × 423)
n = (0.947 × 0.085) / (0.0821 × 423)
n = 0.0023 mole
Finally, we shall obtain the molar mass of the gas. This is shown below:
Mass of gas = 0.250 gNumber of mole of gas = 0.0023 mole Molar mass of gas = ?Molar mass = mass / mole
Molar mass of gas = 0.250 / 0.0023
Molar mass of gas = 108.7 g/mol
Thus, the molar mass of the gas collected is 108.7 g/mol
Learn more about molar mass:
https://brainly.com/question/18983376
#SPJ1
which of the following pairs are ionic compounds ( Al,Cl),(Na,O),(Al,F)
Answers
Answer:
This answer I think it is Al,F
Which of the following statements concerning protein domains is true?
a. They are a form of secondary structure.
b. They are examples of structural motifs.
c. They consist of separate polypeptide chains (subunits). (b) pH = 12.0
d. They have been found only in prokaryotic proteins.
e. They may retain their correct shape even when separated from the rest of the protein.
Answers
The correct statement is statement (e). As per the statement, they may retain their correct shape even when it is separated from the rest of the protein.
Protein is located in the course of the frame—in muscle, bone, skin, hair, and virtually each other body component or tissue. It makes up the enzymes that power many chemical reactions and the hemoglobin that carries oxygen for your blood.
Excessive protein vegetables are greens that offer an excellent amount of protein in your diet. This includes legumes (a category of greens) and greater traditional vegetables like lima beans, inexperienced peas, spinach, sweet corn, artichokes, Brussels sprouts, candy potato, asparagus, broccoli, kale, mushrooms and avocado.
Learn more about Protein:
brainly.com/question/10058019
#SPJ4
What is the pH of a solution with a [H3O+] concentration of 3.4 x 10-¹¹ M?
Answers
The pH of the solution will be 10.47.
what is pH?The pH of a solution is mathematically given as:
pH = - log [\(H^+\)] of -log [\(H_3O^+\)]
Thus, in this case, with [\(H_3O^+\)] of 3.4 x 10-¹¹ M:
pH = -log 3.4 x \(10^-^1^1\) = 10.47
Thus, the pH of the solution will be 10.47.
More on pH can be found here: https://brainly.com/question/15289741
#SPJ1
what type of reaction takes place between methane and chlorine
Answers
Answer and Explanation:
When methane (CH4) and chlorine (Cl2) react, a substitution reaction takes place. Specifically, the reaction is a halogenation reaction, in which one or more hydrogen atoms in the methane molecule are replaced by chlorine atoms. The reaction can be represented by the following equation:
CH4 + Cl2 → CH3Cl + HCl
In this equation, CH3Cl represents chloromethane, which is a type of organochlorine compound. The reaction is typically initiated by ultraviolet (UV) light or heat, and it proceeds through a series of free radical chain reactions. The products of the reaction are typically a mixture of chloromethane and hydrogen chloride (HCl) gas.
What is the molarity of a 50.0ml aqueous solution containing 10.0 grams of hydrogen peroxide H2O2
Answers
Molarity= No of moles of solute * 1000 / vol solution in ml
No of moles= Given mass / Molar mass
Given Mass of solute (H2O2)= 10g
Molar mass of H2O2=34gmol^-1
No of moles= 10/34= 0.294 moles
Volume of solution=50ml
Molarity = 0.294*1000 / 50
Molarity = 5.8M
Characteristics of an atom
Answers
Answer:
It is composed of protons, which have a positive charge, and neutrons, which have no charge. Protons, neutrons, and the electrons surrounding them are long-lived particles present in all ordinary, naturally occurring atoms. Other subatomic particles may be found in association with these three types of particles.
Explanation:
Atoms consist of three basic particles: protons, electrons, and neutrons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).

please helppp!!
How do ion charges differ
for the fourth main group
column and the transition
metals?
Answers
Answer:
How do ion charges differ for the fourth main group column and the transition metals? Do not have common charges. Transition metals tend to form positive ions, but the number of electrons that are lost varies. Describe how atoms with a large difference in electronegativity form ionic bonds.
Explanation: ?
What type of mixture is fog?
O A. A homogenous mixture of a solid in a gas
B. A heterogeneous mixture of a liquid in a gas
C. A homogenous mixture of a liquid in a gas
D. A heterogeneous mixture of a solid in a gas
Answers
Answer:
. A homogeneous mixture of a solid in a gas