a buffer with a ph of 9.85 contains ch3nh2 and ch3nh3cl in water. what can you conclude about the relative concentrations of ch3nh2 and ch3nh3cl in this buffer? for ch3nh2, pkb
Answers
The solution will be a basic buffer.
The pH should be within 1 pH unit of the weak acid's pKa. This means that the acid and base concentrations should not differ by more than 10-fold. A buffer is a solution that can withstand changes in pH due to the addition of acid or base components. It can neutralize small amounts of added acids or bases and keep the pH of the solution relatively stable.
This is important for processes and reactions that require a specific stable pH range. Buffers maintain a stable pH by neutralizing added acids or bases. They are composed of weak acids and their conjugate bases, which exchange protons and hydroxide ions to form water. The Henderson-Hasselbalch equation describes pH as a function of PKA and the ratio of base concentration to acid concentration.
Learn more about The relative concentrations here:-https://brainly.com/question/10838453
#SPJ4
Related Questions
If oil and water have opposite intermolecular forces, why do they seem to mix at the border between them?
Answers
Answer:
The Chemistry Explanation
Oil and water don't mix because water molecules are not attracted to oil molecules. When the Alka-Selzer tablet is dropped in the oil and water, it sinks to the bottom because it is more dense than oil and more dense than the water.
Intermolecular forces such as dipole-dipole forces, London dispersion forces exist between molecules and these depend on strength of electronegativity of molecule. Therefore, oil and water mix at the border.
What is intermolecular forces of attraction?Intermolecular forces of attraction is force of attraction that make two molecule come closer. Intermolecular forces of attraction is directly proportional to the electronegativity of the molecule.
Size of electronegative molecule is small so, if any other molecule or molecule come into contact with this molecule then this molecule attract the electron of other molecule very efficiently and therefore the force between the molecule increases.
Oil has weaker intermolecular forces than water. This makes the intermolecular forces in oil easier to break, and keeps the molecules stuck together in a liquid state.
Therefore, oil and water mix at the border.
To learn more about intermolecular forces of attraction, here:
https://brainly.com/question/26701678
#SPJ2
1. What class of drugs are being investigated in this study, and how do they get into our waterways? 2. What is a C-start and why is it important for larval fish survival? 3. What hypotheses are being tested in this investigation? 4. Briefly describe what the researchers found when they exposed larval fathead minnows to levels of antidepressants found in our waterways.
Answers
The effects of exposure were more pronounced in fish that had been raised in a less stressful environment, suggesting that environmental conditions can influence the impact of exposure to antidepressants.
1. The class of drugs being investigated in this study is antidepressants. They enter our waterways through excretion by individuals taking the medication, and disposal of unused medication into toilets or sinks that are connected to wastewater treatment plants.
2. C-start is an evasive maneuver that young fish use when they perceive a predator. This is important for larval fish survival because it helps them to avoid being eaten by predators.
3. In this investigation, researchers are testing two hypotheses. The first is that exposure to low levels of antidepressants can affect larval fathead minnows' behavior, and the second is that the effects of exposure will be more pronounced in fish that have been raised in a less stressful environment.
4. The researchers found that exposure to antidepressants at levels found in waterways can have a significant impact on the behavior of larval fathead minnows. Specifically, they found that the fish exposed to antidepressants were less likely to respond to the presence of predators, which could increase their risk of being eaten.
They also found that the effects of exposure were more pronounced in fish that had been raised in a less stressful environment, suggesting that environmental conditions can influence the impact of exposure to antidepressants.
To learn more about antidepressants,
https://brainly.com/question/28209828
#SPJ4
please help!!
How many moles of NO2 are equivalent to 74.3 grams of NO2?
(N = 14.01 g/mol, O = 16.00 g/mol)
Answers
Answer:
= 1.615 moles
Explanation:
I did it on a test and got it right
Pure water has a boiling point of 100°C and a freezing point of 0°C.
What is the boiling point and freezing point of a sample of aqueous sodium chloride?
A3
А
B
C
D
boiling point/°C
98
98
102
102
freezing point/°C
-2
2
-2
2
Answers
liquid water For example, the limited temperature range of liquid water (0°C–100°C) severely limits its use. Aqueous solutions have both a lower freezing point and a higher boiling point than pure water.
What is temperature ?How hot (or energetic) a substance or radiation is can be quantified by a physical value called temperature.
There are three different types of temperature scales: those like the SI scale that are defined in terms of the average translational kinetic energy per freely moving microscopic particle, like an atom, molecule, or electron in a body; those that only rely on strictly macroscopic properties and thermodynamic principles, like Kelvin's original definition; and those that are defined by actual empirical properties of particulates rather than by theoretical principles.
Temperature is gauged using a thermometer. It is calibrated using a variety of temperature scales that historically defined themselves using various reference points and thermometric materials. The most widely used scale is the Celsius scale, previously called as "centigrade."
To learn more about temperature from the given link:
https://brainly.com/question/24746268
#SPJ4
explain why measuring the concentration of harmful chemicals in the air is more accurate than estimating the amount of harmful chemicals in emissions
Answers
The measuring of the concentration of harmful chemicals in the air is more accurate than estimating the amount of harmful chemicals in emissions because the molar concentration can be traced up to ppb, ppm levels.
What is molar concentration?Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
Learn more about molar concentration,here:
https://brainly.com/question/13385951
#SPJ9
true or false of normal matter, about 25% of it by mass is still primordial helium even today.
Answers
The given statement "of normal matter, about 25% of it by mass is still primordial helium even today." is True. Around 25% of the normal matter in the universe by mass is still primordial helium even today.
Primordial helium is the helium that was formed during the Big Bang nucleosynthesis, which occurred approximately 13.8 billion years ago. This helium has remained unchanged since its formation and accounts for a significant portion of the universe's normal matter, with the majority of the remaining mass being composed of hydrogen.
More on primordial helium: https://brainly.com/question/31932235
#SPJ11
Which of the following has mass?
A. Space
B. Light
C. Matter
Ο Ο
D. Force
Answers
Answer:
matter should be the answer
Explanation:
Hope this helps!
Answer:
matter because matter makes up everything in the world EVERYTHING like this computer ri am typing on? matter. what ever device you are using to view this? matter. everything is made of matter. matter is starting to sound weird now so I'm gonna stop saying it and this is the end of the answer remember THAT ANSWER IS MATTERR!!!!!
The ranking according to a decreasing ionic radius is thus A s 3 − , S e 2 − , B r − , R b + , S r 2 + .
Answers
The ranking according to a decreasing ionic radius is as follows: Rb+ > Sr2+ > Se2- > As3- > Br-
The term ionic radius is the distance between the nucleus and the outermost electron in an ion. The ionic radius decreases when an atom loses an electron and becomes a cation or gains an electron and becomes an anion. A cation has a smaller radius than its neutral atom because there are fewer electrons and thus a smaller electron cloud. An anion has a bigger radius than its neutral atom since there are more electrons and a bigger electron cloud. The higher the electric charge on an ion, the smaller its radius. The greater the quantity of electrons in an ion, the larger its radius.
The ranking according to a decreasing ionic radius is as follows: Rb+ > Sr2+ > Se2- > As3- > Br-
Explanation: Rb+ is a cation, so its radius is less than that of the Sr atom. Sr2+ has a lower radius than Se2-, which has a lower radius than As3-, which has a lower radius than Br-.
To learn more about ranking visit;
https://brainly.com/question/32670801
#SPJ11
Please help due today
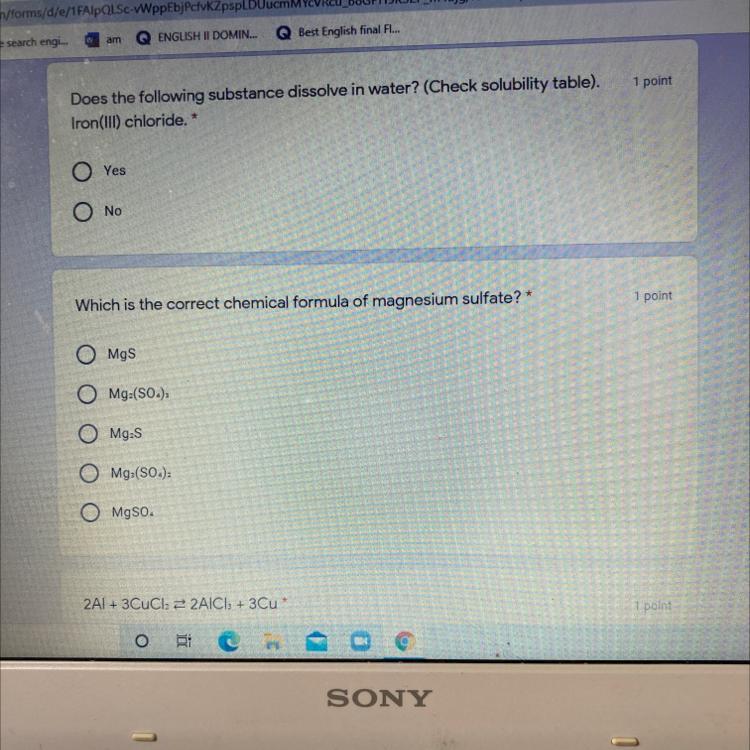
Answers
Answer:
the answer for the magnesium sulphate is MgSO4
Answer:
For the first one it's no.
On your Second question it's MgS.
State the factors that supports
rusting.
Answers
Answer:
water and oxygen
Explanation:
only iron and iron alloys can rust whereas other elements corrode. for rusting to happen, 2 substances need to be present: water and oxygen
Answer:
Metal[Iron]+Water+air[Oxygen]-->Rusting
Explanation:
Rusting is an oxidation reaction.The iron reacts with oxygen and water to form hydrated iron[III]oxide.which is seen as rust due to the change in color [to brown]
Which group has 1 valence electron?

Answers
Answer:
alkali metals
Explanation:
alkali metals are in group 1 (the first column of elements) indicating they have 1 valence electron. halogens are in group 17 and they have 7 valence electrons.
Answer:
A.. Alkali metals
Explanation:
took the quiz
Which term represent a phase change ?
Burning
Melting
Expanding
Cutting
Answers
We went over this in school earlier in the year.
Hope this helps you have a great day
Good luck
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
Ozone is a necessary, protective component of the ________, but is considered a pollutant in the ________.
Answers
Ozone is a necessary, protective component of the Earth's atmosphere, but is considered a pollutant in the troposphere.The Earth's atmosphere is divided into five layers. These layers are known as the troposphere, stratosphere, mesosphere, thermosphere, and exosphere.
Ozone is a protective component of the Earth's atmosphere, but only in the stratosphere. In the troposphere, ozone is considered to be a pollutant.Ozone can be beneficial in the stratosphere because it prevents harmful ultraviolet rays from reaching the Earth's surface. It serves as a protective layer around the Earth, which helps to protect people from the harmful effects of the sun. In contrast, in the troposphere, ozone is a harmful pollutant that is formed by pollutants from cars, power plants, and factories. It is the main component of smog, which can cause a variety of health problems. Smog can be harmful to the respiratory system, and can cause a variety of other health problems as well.
Learn more about Ozone here ;
https://brainly.com/question/14330630
#SPJ11
HURRY WILL MARK BRAINLIEST An unknown substance weighing 34.7 g was heated from 10.2°C to 68.9°C. In this process, the substance absorbed 788 J of energy. What is the specific heat of the substance? Include both the work for the calculation and the answer with appropriate units.
Answers
why are viruses referred to as being obligate parasites ?
Answers
can barium ionically bond with kalium
Answers
Answer:
NO
Explanation:
Barium cannot ionically bond with Kalium. Kalium is the Latin name for potassium.
Both Barium and potassium are metals. Metals and Metals do not combine to form ionic compounds. Only metals and non-metals form this bond type.
The reason for this is that ionic bond forms by the transfer of electrons from one specie to another that receives it. Barium is metal and would freely donate electrons to attain stability. Kalium is metal and shares similar property.
What amount of a 70% acid solution must be mixed with a 20%
solution to produce 200 mL of a 45% solution?
Answers
Answer:
To determine the amount of a 70% acid solution and a 20% solution needed to produce a 45% solution, we can set up a system of equations based on the principles of concentration and volume.
Let's assume that x mL of the 70% acid solution needs to be mixed with (200 - x) mL of the 20% solution.
The total amount of acid in the resulting mixture can be calculated as follows:
0.70x + 0.20(200 - x) = 0.45(200)
Now, let's solve this equation to find the value of x:
0.70x + 40 - 0.20x = 90
0.70x - 0.20x = 90 - 40
0.50x = 50
x = 50 / 0.50
x = 100
Therefore,
100 mL of the 70% acid solution needs to be mixed with (200 - 100) = 100 mL of the 20% solution to produce 200 mL of a 45% solution
To know more about to find amounts of mixed solutions visit:
https://brainly.com/question/33531478
#SPJ11
issued this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your etext. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon.
Answers
The atomic ratio of carbon to oxygen in carbon monoxide (CO) is 1:1, and the atomic ratio of carbon to oxygen in carbon dioxide (CO₂) is 2:1.
Firstly, we can analyze the decomposition of carbon monoxide (CO) and carbon dioxide (CO₂) to determine the atomic ratios involved.
Let's denote the atomic ratio of carbon to oxygen in carbon monoxide as x, and the atomic ratio of carbon to oxygen in carbon dioxide as y.
According to the given data;
Decomposition of carbon monoxide (CO);
Oxygen produced = 3.36 g
Carbon produced = 2.52 g
We know that the atomic mass of carbon is 12 g/mol, and the atomic mass of oxygen is 16 g/mol. Using these values, we can calculate the number of moles for each element;
Number of moles of oxygen = mass / atomic mass = 3.36 g / 16 g/mol = 0.21 mol
Number of moles of carbon = mass / atomic mass = 2.52 g / 12 g/mol = 0.21 mol
Since the atomic ratio of carbon to oxygen in carbon monoxide is x, we can write the following equation;
0.21 mol C / (0.21 mol O) = x
Simplifying the equation, we have;
x = 1
Therefore, the atomic ratio of carbon to oxygen in carbon monoxide is 1:1.
Decomposition of carbon dioxide (CO₂);
Oxygen produced = 9.92 g
Carbon produced = 3.72 g
Following the same calculations as before;
Number of moles of oxygen = mass / atomic mass = 9.92 g / 16 g/mol = 0.62 mol
Number of moles of carbon = mass / atomic mass = 3.72 g / 12 g/mol = 0.31 mol
Since the atomic ratio of carbon to oxygen in carbon dioxide is y, we can write the following equation;
0.31 mol C / (0.62 mol O) = y
Simplifying the equation, we have;
y = 0.5
Therefore, the atomic ratio of carbon to oxygen in carbon dioxide is 1:0.5, which can be simplified to 2:1.
To know more about decomposition here
https://brainly.com/question/20418092
#SPJ4
--The given question is incomplete, the complete question is
"Missed this? watch kcv: atomic theory; read section 2.3. you can click on the review link to access the section in your text. carbon and oxygen form both carbon monoxide and carbon dioxide. when samples of these are decomposed, the carbon monoxide produces 3.36 g of oxygen and 2.52 g of carbon, while the carbon dioxide produces 9.92 g of oxygen and 3.72 g of carbon. Calculate the atomic ratio of carbon to oxygen in carbon monoxide, and carbon dioxide."--
can anyone pls answerr

Answers
Answer:
3. J.J. Thomson & electron
4. b) nucleus
5. neutrons & protons
6. electrons
Which of the following is characteristic of a person with anorexia?
Eating a lot of food.
Eating very little food.
Vomiting after eating.
Abusing laxatives.
Answers
Answer:
Vomiting after eating
Explanation:
Answer:
Physical signs and symptoms of anorexia may include:
Extreme weight loss or not making expected developmental weight gains.Thin appearance.Abnormal blood counts.Fatigue.Insomnia.Dizziness or fainting.Bluish discoloration of the fingers.Hair that thins, breaks or falls outSo it the answer would be :
Eating very little food.how much 6.01 m naoh must be added to 430.0 ml of a buffer that is 0.0180 m acetic acid and 0.0260 m sodium acetate to raise the ph to 5.75?
Answers
We need to add 0.43 moles of NaOH to the buffer. This is equivalent to adding 2.59 L of 6.01 M NaOH to the buffer.
To calculate the amount of 6.01 M NaOH required to raise the pH of the buffer, we first need to determine the current pH of the buffer. Using the Henderson-Hasselbalch equation, we can calculate that the pH of the buffer is 4.76. To raise the pH to 5.75, we need to add enough NaOH to increase the [OH-] concentration by a factor of 10.
This means we need to add 10 times the amount of H+ ions in the buffer solution. From the balanced chemical equation for the ionization of acetic acid, we know that for every mole of acetic acid that ionizes, it produces one H+ ion. Therefore, we need to add 0.43 moles of NaOH to the buffer. This is equivalent to adding 2.59 L of 6.01 M NaOH to the buffer.
To know about buffer:
https://brainly.com/question/31847096
#SPJ11
In fermentation, what do cells use instead of oxygen to break down glucose?
Answers
Answer:
sugar
Explanation:
In the absence of oxygen, the cell uses a process called anaerobic fermentation. or simply fermentation. Fermentation doesn't break the sugar down any further, it simply helps reset the system so that more sugar can be broken down.
Thus the above response is right.
Learn more about fermentation here:
https://brainly.com/question/16516092
CHE505 REACTION ENGINEERING II b) Identify a production using fermentation process. With the aid of simple sketch, briefly explain the process of gas-liquid mass transfer in cellular system for the fermentation process selected. List reference(s) with proper citations.
Answers
An example of a production using fermentation process is the production of ethanol through yeast fermentation.
Yeast fermentation is a commonly used process to produce ethanol from various carbohydrate sources, such as sugars or starches. The fermentation process involves the conversion of sugars into ethanol and carbon dioxide by yeast cells, specifically Saccharomyces cerevisiae.
Gas-liquid mass transfer in the cellular system for yeast fermentation occurs through the following steps:
Oxygen Transfer: In the beginning of the fermentation process, oxygen is required for the growth and metabolism of yeast cells. Oxygen is transferred from the gas phase (air) to the liquid phase (fermentation broth) through mass transfer. This is typically achieved by bubbling air or oxygen-enriched gas into the fermentation vessel. Oxygen dissolves into the liquid and becomes available for yeast cells to utilize.
Carbon Dioxide Release: As yeast cells metabolize the sugars, they produce ethanol and carbon dioxide as byproducts. The carbon dioxide is released into the gas phase, causing the formation of gas bubbles within the liquid. The gas bubbles rise to the surface of the fermentation broth, where carbon dioxide is released into the atmosphere.
The gas-liquid mass transfer in yeast fermentation is crucial for maintaining adequate oxygen levels for yeast growth and removing the produced carbon dioxide from the fermentation broth. Efficient mass transfer ensures optimal yeast activity and ethanol production.
References:
Chen, X., Nielsen, K. F., & Borodina, I. (2014). Kiwi yeast-like endophytes produce xylitol. Microbial cell factories, 13, 112. doi: 10.1186/s12934-014-0112-2
Grigoras, C. G., Moraru, L., & Popescu, C. (2019). Bioethanol production from biomass: overview of biomass conversion technologies. In Advanced technologies for the valorization of biomass, waste, and byproducts (pp. 49-80). Elsevier. doi: 10.1016/B978-0-12-818762-0.00002-4
The production of ethanol through yeast fermentation is an example of a production process using fermentation. Gas-liquid mass transfer in this cellular system involves the transfer of oxygen from the gas phase to the liquid phase for yeast growth and the release of carbon dioxide into the gas phase as a byproduct of yeast metabolism. Efficient mass transfer is essential for optimal fermentation performance and ethanol production. The provided references can be consulted for further information and citations.
To know more about ethanol visit,
https://brainly.com/question/5750283
#SPJ11
Which of the following is a liquid?
milk
oxygen
cheese
Answers
A 1.8−mL volume of seawater contains about 4.0 × 10^−10 g of gold. The total volume of ocean water is about 1.5 × 10^21 L. Calculate the total worth of all the gold in the world's oceans if the price of gold is $19.96 per gram answer is in scientific notation
Answers
Answer: $6.653×10^15
Explanation:
Volume of sea water sample = 1.8mL
Mass of gold in sample = 4.0 × 10^-10 g
Total volume of ocean water = 1.5 × 10^21 L
Converting volume of seawater sample into liters :
1.8mL = (1.8/1000)L = 0.0018L = 1.8×10^-3
If volume of gold in ;
1.8×10^-3L = 4 × 10^-10 g
1.5×10^21 L = X
(1.8×10^-3L)X = [(1.5×10^21) × (4×10^-10) ]
X = [1.5×4(10^21-10)] / 1.8×10^-3
X = 6.0(10^11) / 1.8×10^-3
X = 3.333×(10^11+3)
X = 3.333 × 10^14
Cost of world's gold equals
(3.333 × 10^14) × $19.96
$66.53 × 10^14
I rounded the answer but can you help me?

Answers
3(Cu)+8(O)+2(P)
3(63.546)+8(15.999)+2(30.974) = 380.578g/mol
And then we use sig figs which makes us round to 5 sig figs and gives us 380.58 g/mol
what causes metamorphic rocks to form
Pls help
Answers
Explanation:
Metamorphic rocks form when rocks are subjected to high heat, high pressure, hot mineral-rich fluids or, more commonly, some combination of these factors. Conditions like these are found deep within the Earth or where tectonic plates meet.
5 moles of oxygen gas is equal to how many grams?
Answers
Answer:
160 g
Explanation:
1 mole of oxygen atom = 16 g
Number of moles of oxygen given = 5 mole
Molecular weight of oxygen molecule = 32 g/ mole
Weight of oxygen in gram = 5 mol x 32 g/mol= 160 g
Therefore, 160 g of oxygen is present in 5 moles.
How to convert 12g of H2O to moles
Answers
Answer:
moles = given mass/atomic mass
so H2O mass = 2 +16=18
so 12g of h2o= 12/16 = 3/4 moles