A. Calculate the molarity ( M ) of 154.1 g of H2SO4 in 1.475 L
of solution. Express your answer to four significant figures.
Answers
The molarity (M) of 154.1 g of H2SO4 in 1.475 L of solution is X.XXXX M, expressed to four significant figures.
Molarity (M) is defined as the number of moles of solute per liter of solution. To calculate the molarity of H2SO4, we need to determine the number of moles of H2SO4 and divide it by the volume of the solution in liters.
1. Calculate the number of moles of H2SO4 by dividing the given mass by its molar mass. The molar mass of H2SO4 is 98.09 g/mol.
Number of moles of H2SO4 = 154.1 g / 98.09 g/mol.
2. Convert the given volume of the solution to liters. The volume is given as 1.475 L.
3. Finally, divide the number of moles of H2SO4 by the volume of the solution in liters to obtain the molarity.
Molarity (M) = Number of moles of H2SO4 / Volume of solution in liters.
Performing the calculations above will give you the molarity of H2SO4 in the given solution, expressed to four significant figures.
To know more about molarity click here:
https://brainly.com/question/2817451
#SPJ11
Related Questions
2. A copper wire can conduct electricity whether the wire is
very thin or very thick. Is electrical conductivity an
extensive property or an intensive property?
Answers
Answer:
Intensive
Explanation:
The electrical conductivity of a wire depends on its composition, not the length of the wire.
Hope this helps! If correct pls mark brainliest :)
As copper wire was very thin, it will have great electrical conductance and copper wire has a intensive property.
What is electrical resistance of wire and how copper wire has intensive property?The electrical resistance of a wire is given by
R = ρl/A.
where,
R -Resistivity of the material,
l - length of the wire,
A - cross-sectional area of the wire
As we know the formula, it can be explained as:
The resistance is proportional to the length of the wire.The resistance is inversely proportional to the cross-sectional area. If the resistance is less then it means that it has the cross - sectional will be large and if the resistance is higher then the cross - sectional area will be small.Thick wires has larger cross sectional area and thin wires has small cross sectional area.Hence, we came to know that, thin wires have a greater electrical resistance than a thick wire.
So, copper wire was very thin and it can conduct electricity.
An intensive property is a property of matter that depends on the type of matter of the sample not considered by the amount of matter. Other intensive properties include color, temperature, density, and solubility.Here, concluded that copper wire was very thin and it has intensive property.
Learn more about electrical conductivity of thin wire,
https://brainly.com/question/14074813
#SPJ2
Explain why mixtures can be separated by physical methods, such as sieving and distillation.
Answers
True or false - Evaporating, condensing, melting and freezing are all examples of chemical change.
Answers
Answer:
false
Explanation:
Because these examples are able to turn back into their original form. For example, melted ice can refreeze.
Draw the best Lewis structure for CCl3^-1 What is the formal charge on the C?
Answers
According to this, formal charge on C in best Lewis structure of CCl3+1 is +1.
The formal charge is the real charge that an atom carries when it is a part of a bigger molecule or polyatomic ion.
The net overall charge is the consequence of the formal charges on each molecule or ion added together.
For tiny ions, this idea is understandable. It is clear that chloride has a negative charge. Even understanding the negative charge on the hydroxide oxygen is straightforward.
Formal Charge = (# valence electrons on neutral atom) - (# lone electron pairs) + (12 # bonding electrons) Valence electrons = according to group number of periodic table (for representative elements). Lone Pairs are atoms with just one electron in each. One electron makes up a pair, which makes two.
Learn more about Lewis structure here:
https://brainly.com/question/20300458
#SPJ4
PLEASE HELP!!!!
There are a lot of misconceptions about evolution. Using what you have learned in this movie, counter each of the following myths with a factual argument. You may use the Internet or other resources for additional information.

Answers
Answer:
Given its huge success in describing the natural world for the past 150 years, the theory of evolution is remarkably misunderstood. In a recent episode of the Australian series of “I’m a Celebrity Get Me Out of Here”, former cricket star Shane Warne questioned the theory – asking “if humans evolved from monkeys, why haven’t today’s monkeys evolved”?
Similarly, a head teacher from a primary school in the UK recently stated that evolution is a theory rather than a fact. This is despite the fact that children in the UK start learning about evolution in Year 6 (ten to 11-year-olds), and have further lessons throughout high school. While the theory of evolution is well accepted in the UK compared with the rest of the world, a survey in 2005 indicated that more than 20% of the country’s population was not sure about it, or did not accept it.
Explanation:
Answer:
Humans did not evolve from monkeys
Explanation:
Us humans and apes had a common ancestor that split in path millions of years ago. This resulted in our ancestor and ape ancestor. Monkeys may of came from ape, but we definitely did not evolve from monkey
how would the acetic acid/acetate buffer system neutralize an added base?
Answers
The acetic acid/acetate buffer system consists of a weak acid (acetic acid, CH3COOH) and its conjugate base (acetate ion, CH3COO-). When a base is added to the buffer system, the following process occurs to neutralize it:
1. The base reacts with the weak acid (acetic acid) in the buffer system to form its conjugate base (acetate ion) and water. For example, if a hydroxide ion (OH-) is added, it reacts with acetic acid as follows:
OH- + CH3COOH → CH3COO- + H2O
2. The conjugate base (acetate ion) that is formed acts as a reservoir for hydrogen ions (H+). It can accept hydrogen ions from the solution if the pH increases. This helps to maintain the pH of the buffer system within a certain range.
3. The buffer system resists large changes in pH because the equilibrium between the weak acid and its conjugate base is shifted to maintain a relatively constant concentration of both species. This allows the system to neutralize the added base and maintain its acidic nature.
The acetic acid/acetate buffer system neutralizes an added base by reacting with it to form the conjugate base and water, and by utilizing the conjugate base to accept hydrogen ions and maintain the pH of the system.
To learn more about acetic acid, visit:
brainly.com/question/24586675
#SPJ11
list the processes that release carbon into the atmosphere
Answers
Answer:
Fossil fuels, such as coal, oil, or natural gas, release carbon back into the atmosphere.
The processes would be decomposition, diffusion, erosion, respiration, and combustion.
Explanation:
Hope this helped?
in water and ice, the slightly hydrogen atoms in one water molecule are drawn to the slightly oxygen atom in another water molecule.
Answers
In water and ice, the hydrogen atoms within one water molecule experience a weak attractive force towards the oxygen atom in a neighboring water molecule.
This intermolecular force is known as hydrogen bonding. Hydrogen bonding occurs due to the difference in electronegativity between oxygen and hydrogen atoms.
In a water molecule (H2O), oxygen is more electronegative than hydrogen. As a result, the oxygen atom tends to attract the shared electrons in the covalent bond more strongly, creating a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms.
The partially positive hydrogen atoms can interact with the partially negative oxygen atoms of nearby water molecules through hydrogen bonding. The δ+ hydrogen atom from one water molecule is attracted to the δ- oxygen atom of another water molecule. This attraction occurs because the positively charged hydrogen atom is strongly attracted to the negatively charged oxygen atom.
Hydrogen bonding between water molecules contributes to the unique properties of water, such as its high boiling point, surface tension, and ability to dissolve many substances. In the solid form of water, known as ice, the hydrogen bonds between water molecules are stable and form an open lattice structure, leading to the characteristic hexagonal arrangement of ice crystals.
learn more about hydrogen here
https://brainly.com/question/30623765
#SPJ11
A. How many protons, electrons, and neutrons make up an atom
of bromine-80?
Mass number of bromine-80:
Atomic number of bromine:
Number of protons:
Number of electrons:
Number of neutrons =__-__=__
Answers
Answer:
45 neutrons
Explanation:
Bromine has 35 protons and a mass number of 80. a) How many neutrons does the atom of bromine have? The mass number = protons + neutrons. Bromine has a mass number of 80 and 35 protons so 80-35 = 45 neutrons.
Electrons, protons, and neutrons are subatomic particles. In Bromine number of protons is 35, electrons are 35, and neutrons are 45.
What are subatomic particles?Subatomic particles are the electrons, protons, and neutrons that are found in the atom. The number of electrons and protons is generally equal in the case of a neutral atom.
The atomic number of the Bromine atom is 35 and thus, protons are 35 and so are electrons.
The number of neutrons is given as the difference between the atomic mass and proton.
The number of neutrons are:
= Atomic mass - protons
= 80 - 35
= 45
The number of neutrons is 45, and the number of protons and electrons is 35.
Learn more about subatomic particles here:
https://brainly.com/question/8419082
#SPJ2
what is -56 0C to 0F
Answers
-56 degree C when converted to degree F will be equal to -68.8 degree F.
What is degree?Degree is defined as a unit of measurement that is used to measure angles, and also longitude and latitude.
The Celsius scale start from 0 while the Fahrenheit scale start from 32 degree F which is equal to 0 degree C.
On the basic of the concept the formula is
F = 9/5 C + 32
Thus by using formula we get
F = 9/5(-56) + 32
F = 9(-11.2) + 32 = -100.8 + 32
F = -68.8
Thus, the correct answer of -56 degree C to °F is -68.8 degree F.
To learn more about degree ,refer to the link below:
https://brainly.com/question/364572
#SPJ1
50 POINTS! I NEED HELP ASAP PLEASE WILL MAKR BRAINLIEST
Jackson noticed that the music from his cell phone sounds louder when laid on his desk than when he held it up in the air. Which of the following explains the difference in sound that Jackson is experiencing?
Sound waves move faster through air because the molecules are further apart.
Sound waves move faster through solids because the molecules are further apart.
Sound waves move faster through air because the molecules are closely packed together.
Sound waves move faster through solids because the molecules are closely packed together.
Answers
Jackson noticed that the music from his cell phone sounds louder when laid on his desk than when he held it up in the air. Sound waves move faster through solids because the molecules are closely packed together. Therefore, option D is correct.
What is sound wave ?When energy moves through a medium and propagates away from the sound source, it creates a pattern of disruption known as a sound wave. Pressure waves are generated by the vibration of objects and are known as sound waves.
When energy forces air molecules to shift in and out of one another, sound is produced. The sound's amplitude increases as the particles approach closer or farther apart. A sound's volume and intensity are determined by its amplitude. The sound gets louder and more intense the larger the amplitude.
The results of the testing demonstrated that Solid was the best medium out of the three for sound to travel loudest. The liquid medium came in second. The least effective medium was air.
Thus, option D is correct.
To learn more about sound wave, follow the link;
https://brainly.com/question/21995826
#SPJ1
L. Complete this nuclear equation for the alpha decay of Uus-294 by writing a
notation for the missing product:

Answers
The nuclear equation for the alpha decay of Uus-294 is
\(^{294}{117} Uus\) →\(^{4}{2}He + ^{290}_{115}Mc\)
To complete the nuclear equation for the alpha decay of Uus-294, we need to determine the missing product.
During alpha decay, the emission of an alpha particle, composed of two protons and two neutrons, leads to the formation of a new nucleus.
The balanced nuclear equation for the alpha decay of Uus-294 can be represented as follows:
\(^{294}{117} Uus\) → \(^{4}{2}He +\)_____(missing product)
In this equation, the atomic number of the missing product must be two less than the atomic number of Uus-294 (117 - 2 = 115), and the mass number of the missing product must be four less than the mass number of Uus-294 (294 - 4 = 290).
Based on this information, the missing product can be identified as:
\(^{290}_{115}Mc\)
Mc stands for Moscovium, which has an atomic number of 115. By subtracting two from the atomic number of Uus, we obtain the atomic number of Mc. The mass number of Mc-290 is obtained by subtracting four from the mass number of Uus-294.
Therefore, the nuclear equation for the alpha decay of Uus-294 is:
\(^{294}{117} Uus\)→ \(^{4}{2}He + ^{290}_{115}Mc\)
Know more about alpha decay here:
https://brainly.com/question/25714324
#SPJ8
The complete question is :
Complete this nuclear equation for the alpha decay of Uus-294 by writing a
notation for the missing product:
\(^{204} {117} Uns\)→\(^{4} _{2} He +\)_____
If an electron is confined in a 10 nm box, calculate
its energy in the ground state and 15t
excited state
If an electron is confined in a 10 nm box, calculate
its energy in the ground state and 1st
excited state
Answers
The energy in the ground state of the electron confined in a 10 nm box is approximately 10.89 eV, and the energy in the first excited state is approximately 43.56 eV.
To calculate the energy of an electron confined in a 10 nm box, we can use the formula for the energy levels of a particle in a one-dimensional infinite potential well:
E_n = (n^2 * h^2) / (8 * m * L^2)
where:
E_n is the energy of the nth energy level,
n is the quantum number of the energy level (n = 1 for the ground state),
h is the Planck's constant (6.626 x 10^-34 J·s),
m is the mass of the electron (9.10938356 x 10^-31 kg),
L is the length of the box (10 nm = 10 x 10^-9 m).
Let's calculate the energy in the ground state (n = 1) and the first excited state (n = 2):
For the ground state (n = 1):
E_1 = (1^2 * h^2) / (8 * m * L^2)
Substituting the values:
E_1 = (1^2 * (6.626 x 10^-34 J·s)^2) / (8 * (9.10938356 x 10^-31 kg) * (10 x 10^-9 m)^2)
Calculating this expression will give us the energy in the ground state.
For the first excited state (n = 2):
E_2 = (2^2 * h^2) / (8 * m * L^2)
Substituting the values:
E_2 = (2^2 * (6.626 x 10^-34 J·s)^2) / (8 * (9.10938356 x 10^-31 kg) * (10 x 10^-9 m)^2)
Calculating this expression will give us the energy in the first excited state.
Please note that the energies calculated will be in joules (J). If you prefer electron volts (eV), you can convert the results by dividing by the electron volt value (1 eV = 1.602 x 10^-19 J).
Performing the calculations:
For the ground state:
E_1 = (1^2 * (6.626 x 10^-34 J·s)^2) / (8 * (9.10938356 x 10^-31 kg) * (10 x 10^-9 m)^2) ≈ 1.747 x 10^-18 J
For the first excited state:
E_2 = (2^2 * (6.626 x 10^-34 J·s)^2) / (8 * (9.10938356 x 10^-31 kg) * (10 x 10^-9 m)^2) ≈ 6.987 x 10^-18 J
Converting the energies to electron volts (eV):
E_1 ≈ 10.89 eV (rounded to two decimal places)
E_2 ≈ 43.56 eV (rounded to two decimal places)
Therefore, the energy in the ground state of the electron confined in a 10 nm box is approximately 10.89 eV, and the energy in the first excited state is approximately 43.56 eV.
Learn more about electron from the given link!
https://brainly.com/question/13998346
#SPJ11
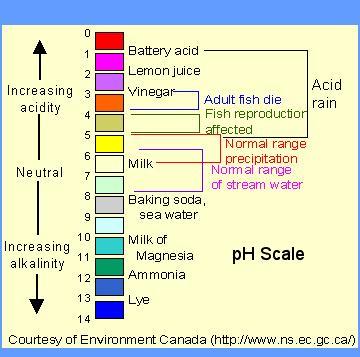
Ph4 is greater than ph6?.
Answers
Answer:
Explanation: For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. The same holds true for pH values above 7, each of which is ten times more alkaline (another way to say basic) than the next lower whole value.
HOPE THAT HELPS
Photosynthesis is an example of an __
process.
Answers
Which one of the following salts when dissolved in water will hydrolyse?
Answers
Option c) NH4Cl will hydrolyze when dissolved in water.
Hydrolysis is a chemical reaction that occurs when a salt reacts with water, resulting in the formation of an acidic or basic solution. In this case, when NH4Cl (ammonium chloride) is dissolved in water, it undergoes hydrolysis.
The ammonium ion (NH4+) is the conjugate acid of ammonia (NH3), which is a weak base. When NH4Cl dissociates in water, the ammonium ion reacts with water to form NH3 and H3O+ (hydronium ion). This process is called hydrolysis.
NH4+ (aq) + H2O (l) ↔ NH3 (aq) + H3O+ (aq)
The formation of NH3 leads to an increase in the concentration of hydroxide ions (OH-) in the solution, making it slightly basic. At the same time, the presence of H3O+ ions makes the solution slightly acidic. Therefore, the hydrolysis of NH4Cl results in a slightly acidic and slightly basic solution.
In contrast, salts like NaCl and KCl do not undergo hydrolysis when dissolved in water because they consist of cations (Na+ and K+) and anions (Cl-) that do not react with water to form acidic or basic species.
Na2SO4 (sodium sulfate) is also an example of a salt that does not undergo hydrolysis. The sulfate ion (SO42-) does not react with water to form acidic or basic species, so the solution remains neutral.
Therefore, among the given options, only NH4Cl undergoes hydrolysis when dissolved in water.
Know more about Hydrolysis here:
https://brainly.com/question/30468294
#SPJ8
The question is incomplete. Find the full content below:
Which one of the following salts when dissolved in water will hydrolyse?
a) NaCl
b) KCl
c) NH4Cl
d)Na2SO4
Due to risk of electrical shock, team member must be careful not to let any water splash into lemonade dispenser while panels are off for cleaning.
a. true
b. false
Answers
b. false Water is a good conductor of electricity, and if it comes into contact with electrical components or wiring, it can create a risk of electrical shock. Therefore, team members should indeed be careful not to let water splash into the lemonade dispenser when the panels are off for cleaning.
This precaution is necessary to ensure the safety of the individuals handling the equipment. It is important to note that electrical shock can occur when there is a complete or partial path for electric current to flow through the body. Water can provide this path of conductivity and increase the likelihood of electrical shock. Therefore, team members should take appropriate precautions to prevent water from reaching the electrical components and ensure their own safety.
Learn more about panels here: brainly.com/question/6949231
#SPJ11
5. A box with a volume of 22.4 L contains 1.0 mol of nitrogen and 2.0 mol of hydrogen at 0°C. Which of the following statements is true?
A. The total pressure is 202 kPa.
B. The partial pressure of N2 is 101 kPa.
C. The total pressure in the box is 101 kPa.
D. The partial pressures of N2 and H2 are equal.
Answers
B. The partial pressure of N2 is 101 kPa
Further explanationGiven
volume = 22.4 L
1.0 mol of nitrogen and 2.0 mol of hydrogen at 0°C
Required
Total pressure and partial pressure
Solution
Ideal gas law :
PV = nRT
n total = 3 mol
T = O °C + 273 = 273 K
P = nRT/V
P = 3 x 0.08205 x 273 / 22.4
P total = 3 atm = 303,975 kPa
P Nitrogen = 1/3 x 303.975 = 101.325 kPa
P Hydrogen = 2/3 x 303.975 = 202.65 kPa
Answer:The partial pressure of N2 is 101 kPa
Explanation:
How many o2 molecules are required for two glucose molecules to undergo cellular respiration.
Answers
Two glucose molecules would require 12O₂ molecules to go through cellular respiration.
What is cellular respiration?Through a sequence of chemical processes called cellular respiration, glucose is broken down to create ATP, which may then be used as an energy source for a variety of bodily functions. The citric acid cycle, oxidative phosphorylation, and glycolysis are the three primary phases of cellular respiration.
Let's first examine the respiration equation in
C₆H₁₂O₆+6O₂ → 6CO₂+ 6H₂O
According to the equation, the reaction requires 6 moles of oxygen for every mole of glucose.
6 x 2 = 12 moles of oxygen will be needed to provide 2 glucose molecules.
To know more about cellular respiration visit:
https://brainly.com/question/19269610
#SPJ4
how would you synthesize the following compounds from butanoic acid using reagents from the table? use letters from the table to list reagents in the order used (first at the left). example: ab
a. 1. NaBH4
2. H3O+
b. 1. LiAlH4
2. H3O
c. H2/Pt
d. 1. BH3/THF
2. H2O2/NaOH
e. PBr3.
Answers
The synthesis of the following compounds from butanoic acid using reagents from the table can be carried out as follows:
a. Synthesis of 3-hydroxybutanoic acid from butanoic acid using NaBH4 and H3O+ reagents. Synthesis scheme: NaBH4, H3O+
b. Synthesis of butan-1-ol from butanoic acid using LiAlH4 and H3O reagents. Synthesis scheme: LiAlH4, H3O
c. Synthesis of butane from butanoic acid using H2/Pt reagents. Synthesis scheme: H2/Pt
d. Synthesis of butan-1-ol from butanoic acid using BH3/THF and H2O2/NaOH reagents. Synthesis scheme: BH3/THF, H2O2/NaOH
e. Synthesis of butyl bromide from butanoic acid using PBr3 reagent. Synthesis scheme: PBr3Therefore, the correct order of reagents used for the synthesis of the given compounds from butanoic acid are:
a. NaBH4, H3O+b. LiAlH4, H3Oc. H2/Ptd. BH3/THF, H2O2/NaOHe. PBr3
To know more about butanoic acid click here:
https://brainly.com/question/10242360
#SPJ11
The nucleus of the cell
a
Is found inside the chloroplast
b
Is found inside the membrane of a bacterial cell
c
contains DNA and controls the cell's activities
Answers
Answer:
c
Explanation:
it is c because that is the andwer
a tank contains 8l of water in which is dissolved 32 g (grams) of chemical. a solution containing 2 g/l of the chemical flows into the tank at a rate of 4 l/min, and the well-stirred mixture flows out at a rate of 2 l/min. determine the amount of chemical in the tank after 20 minutes. show all work and setup.
Answers
A tank contains 8l of water in which is dissolved 32 g (grams) of chemical. a solution containing 2 g/l. The amount of chemical in the tank after 20 minutes is 98.66 g.
Given that:
rate r1 = 4 L/min
rate r2 = 2 L/min
concentration c1 = 2 g/L
V(0) = 8 L
A(0) = 32 g
the equation is given as :
ΔV = r1 Δt - r2Δt
dV/dt = 2
integrating the condition , we get
V(t) = 2(t+4)
ΔA = c1r1 Δt - c2r2 Δt
dA/ dt = 8-2c2
c2 = A/ V
dA/dt = 8 - 2A/ V
now by putting the value of V, we get
dA / dt + 1 / t + 4 A = 8
linear equation has integrating factor :
I = e^ ∫ 1/( t + 4) dt = t + 4
d [ ( t + 4)A] / dt = 8 (t + 4)
(t+ 4 )A = 4(t + 4)² + c
A(t) = (1 / t + 4 ) [ 4(t + 4)² + c]
A(0) = 32 means c = 64
A(20) = (1/6) [(24)² + 16]
= 296 / 3
= 98.66 g
To learn more about rate here
https://brainly.com/question/28034602
#SPJ4
Which best describes why carbocation formation is the rate determining step of a unimolecular substitution reaction? The ABC step is slow. The nucelophile is too weak to attack without a carbocation present. The carbocation formed is unstable and high in energy. Carbocation formation is reversible so it doesn't readily occur. The solvent is acting as the nucleophile.
Answers
The best option that describes why carbocation formation is the rate-determining step of a unimolecular substitution reaction is that The carbocation formed is unstable and high in energy.
A carbocation is a positively charged carbon atom that has a vacant orbital. Carbocations are reactive intermediates that are formed in many organic reactions. Carbocations, also known as carbonium ions, are intermediates that are formed when a carbon atom has a positive charge. A carbocation, in a unimolecular substitution reaction, is the rate-determining step.
This is due to the carbocation's instability and high-energy state. During the carbocation-forming step, the energy barrier is the highest, indicating that the rate-determining step is the carbocation-forming step. Carbocations are highly reactive, making them critical intermediates in many chemical reactions.
They are usually unstable because the carbon atom has only six electrons in its valence shell. The positive charge attracts the electrons in the neighboring bonds, destabilizing the carbocation. The reaction may not proceed efficiently if the carbocation cannot be formed quickly. As a result, carbocation formation is critical for the reaction's effectiveness.
For more question on carbocation click on
https://brainly.com/question/31013261
#SPJ11
When the ranges of two different species meet, a stable hybrid zone occupied by hybrid individuals may form. How is this possible?.
Answers
When the ranges of two different species meet, a stable hybrid zone occupied by hybrid individuals may form. This is possible because hybrid zones are often formed when two previously isolated populations of two different species come together to reproduce, resulting in hybrids.
In a hybrid zone, hybrids are produced when the genes from two different species mix and interact. The hybrids possess a combination of the traits from both parent species, and they can differ from both parent species. The hybrids also have the potential to breed with individuals from either parent species, which is what allows the hybrid zone to remain stable. Hybrid zones can form in a variety of ways. For instance, a hybrid zone may form when two previously isolated populations come into contact due to human activities such as habitat destruction or development. Alternatively, a hybrid zone may form when two different species migrate into an area where they overlap in their ranges. In either case, the hybrid zone can be stable if the hybrids are able to reproduce successfully and form viable populations that can continue to produce hybrids.Hybrid zones can be important for understanding the evolutionary history and genetic diversity of different species. They can also provide insight into the mechanisms of speciation and the factors that contribute to the formation of new species.
To Know more about hybrids visit:
brainly.com/question/29020053
#SPJ11
Explain why the sign of the slope of a regression line must be thesame as the sign of the correlation coeffi cient.
Answers
Answer:
The calculation of a standard deviation involves taking the positive square root of a nonnegative number. As a result, both standard deviations in the formula for the slope must be nonnegative. ... Therefore the sign of the correlation coefficient will be the same as the sign of the slope of the regression line.
Explanation:
have a nice day
HELP! ASAP!!! WILL MARK BRAINLIEST!!
Which of the following compounds in the main group is an alkali metal? Question 7 options: Beryllium (Be) Rubidium (Rb) Hydrogen (H) Strontium (Sr)
Answers
Answer:
Rb
Alkali Metals are Group 1 so
Rb it isnt Hydrogen because it is a gas
Explanation:
1 mole of a gas occupies 22.4 L at ________
Answers
Answer: At STP, one mole (6.02 × 1023 representative particles) of any gas occupies a volume of 22.4 L (Figure below). A mole of any gas occupies 22.4 L at standard temperature and pressure (0°C and 1 atm).
Explanation:
That the answer
1 mole of a gas occupies 22.4 L at (0°C and 1 atm). It is the standard temperature and pressure.
What is Avogadro's hypothesis?Avogadro's hypothesis says that all gases have an equal number of particles at the same temperature and pressure.
The empty space between the particles makes up the majority of a gas's overall volume.
Due to this, the size of the particles themselves is nearly insignificant.
The volume of one mole of a gas at standard temperature and pressure is known as the molar volume.
Thus, the correct option is 0°C and 1 atm.
Learn more about Avogadro's hypothesis
https://brainly.com/question/12779877
A 1.5 L pocket of air with a temperature of 295 K rises in the atmosphere. What will be the volume of the air pocket of the t temperature decreased to 2 celsius and the pressure is not changed.
Answers
Answer:
1.4 L.
Explanation:
Applying Charles law,
V/T = V'/T'....................... Equation 1
Where V = Initial volume, V' = Final volume, T = Initial Temperature in Kelvin, T' = Final Temperature in Kelvin.
Make V' the subject of the equation
V' = (V/T)T'..................... Euqation 2
Given: V = 1.5 L, T = 295 K, T' = 2 °C = (2+273) K = 275 K
Substitute these values into equation 2
V' = (1.5/295)275
V' = 1.398
V' ≈ 1.4 L
Positron emission tomography (pet) imaging relies upon the emission of __________ from radioactive isotopes such as carbon-11.
Answers
Answer:
positron
Explanation:
PET scanning works by injecting a radioactive material, called a tracer, into the body. The tracer emits a positron inside the body, and the positron collides with electrons in the body, thereby annihilating and releasing two gamma rays that travel in opposite directions.
Studies show that
O the more you sit, the higher your risk for depression,
O the less you sit, the higher your risk for depression
O the more you sit, the lower your risk for depression
O none of the above
Answers
Answer:
it will be none of the above because sitting doesn't cause depression, it's the environment around that person. the body language, the way they talk... Etc.