Answers
Answer:
i hope the answer is 12.0
Related Questions
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
A community needs more electrical energy. The community is located in an
area with the following characteristics:
• Average wind speed of 0 to 5 mph
• Few rivers or streams
Rainy conditions most of the year
• Large surplus of waste corn
Based on this information, which energy source would be most economical
for the community to use?
A. Hydroelectric
B. Solar
C. Wind
D. Biomass

Answers
Answer:
D BIOMASS ENERGY
Explanation:
Most biomass requires arable land to develop. This means
that land used for biofuel crops such as corn and soybeans
are unavailable to grow food or provide natural habitats.
Forested areas that have matured for decades (so-called
“old-growth forests”) are able to sequester more carbon
than newly planted areas.
Correctly write the chemical formula for as many ions and compounds as you can:
1. Copper (11) ion
2. Bromide ion
3. Magnesium ion
4. Phosphide ion
5. Copper (11) Bromide
6. Sulfur Dichloride
7. Barium Fluoride
8. Magnesium Phosphate
9. Lithium Permanganate
10. Strontium Sulfite
11. Nitrogen Monoxide
12. Diselenium Tetraoxide
13. Aluminum Sulfide
14. Tin (IV) lodide
15. Beryllium Oxide
16. Potassium Hydroxide
Answers
The chemical formulas for the ions and compounds you listed:
Copper (II) ion: Cu²⁺
Bromide ion: Br⁻
Magnesium ion: Mg²⁺
Phosphide ion: P³⁻
Copper (I) Bromide: CuBr
Sulfur Dichloride: SCl₂
Barium Fluoride: BaF₂
Magnesium Phosphate: Mg₃(PO₄)₂
Lithium Permanganate: LiMnO₄
Strontium Sulfite: SrSO₃
Nitrogen Monoxide: NO
Diselenium Tetraoxide: Se₂O₄
Aluminum Sulfide: Al₂S₃
Tin (IV) Iodide: SnI₄
Beryllium Oxide: BeO
Potassium Hydroxide: KOH
Copper (II) ion: Cu²⁺
Copper (II) ion has a charge of 2+ and is represented by Cu²⁺. This means that copper has lost two electrons, resulting in a 2+ charge.
Bromide ion: Br⁻
The bromide ion has a charge of 1- and is represented by Br⁻. This means that bromine has gained one electron, resulting in a 1- charge.
Magnesium ion: Mg²⁺
The magnesium ion has a charge of 2+ and is represented by Mg²⁺. This means that magnesium has lost two electrons, resulting in a 2+ charge.
Phosphide ion: P³⁻
The phosphide ion has a charge of 3- and is represented by P³⁻. This means that phosphorus has gained three electrons, resulting in a 3- charge.
Copper (I) Bromide: CuBr
Copper (I) bromide is a compound formed by combining copper (I) ion (Cu⁺) and bromide ion (Br⁻). The charges of the ions balance each other, resulting in a neutral compound.
Sulfur Dichloride: SCl₂
Sulfur dichloride is a compound consisting of one sulfur atom (S) and two chlorine atoms (Cl). The subscript "2" indicates the presence of two chlorine atoms.
Barium Fluoride: BaF₂
Barium fluoride is a compound composed of one barium ion (Ba²⁺) and two fluoride ions (F⁻). The charges of the ions balance each other, resulting in a neutral compound.
Magnesium Phosphate: Mg₃(PO₄)₂
Magnesium phosphate is a compound consisting of one magnesium ion (Mg²⁺) and two phosphate ions (PO₄³⁻). The charges of the ions balance each other, resulting in a neutral compound. The subscript "3" indicates the presence of three magnesium ions, and the subscript "2" indicates the presence of two phosphate ions.
Lithium Permanganate: LiMnO₄
Lithium permanganate is a compound composed of one lithium ion (Li⁺) and one permanganate ion (MnO₄⁻). The charges of the ions balance each other, resulting in a neutral compound.
Strontium Sulfite: SrSO₃
Strontium sulfite is a compound consisting of one strontium ion (Sr²⁺) and one sulfite ion (SO₃²⁻). The charges of the ions balance each other, resulting in a neutral compound.
Nitrogen Monoxide: NO
Nitrogen monoxide is a compound composed of one nitrogen atom (N) and one oxygen atom (O). Since the compound does not contain ions, it is represented by its elemental symbols.
Diselenium Tetraoxide: Se₂O₄
Diselenium tetraoxide is a compound consisting of two selenium atoms (Se) and four oxygen atoms (O). The prefix "di-" indicates the presence of two selenium atoms.
Aluminum Sulfide: Al₂S₃
Aluminum sulfide is a compound composed of two aluminum ions (Al³⁺) and three sulfide ions (S²⁻). The charges of the ions balance each other, resulting in a neutral compound. The subscript "
2" indicates the presence of two aluminum ions, and the subscript "3" indicates the presence of three sulfide ions.
Tin (IV) Iodide: SnI₄
Tin (IV) iodide is a compound formed by combining tin (IV) ion (Sn⁴⁺) and iodide ion (I⁻). The charges of the ions balance each other, resulting in a neutral compound.
Beryllium Oxide: BeO
Beryllium oxide is a compound composed of one beryllium ion (Be²⁺) and one oxygen ion (O²⁻). The charges of the ions balance each other, resulting in a neutral compound.
Potassium Hydroxide: KOH
Potassium hydroxide is a compound consisting of one potassium ion (K⁺) and one hydroxide ion (OH⁻). The charges of the ions balance each other, resulting in a neutral compound.
For more question on ions click on
https://brainly.com/question/1310794
#SPJ11
Please help will give brainliest
Perform the following
mathematical operation, and
report the answer to the correct
number of significant figures.
328 x 0.125 = [?]

Answers
Answer: 41.0
Explanation: When you multiply the two numbers you get 41 but you need to have the same amount of significant numbers as the number in the problem with the least significant numbers. I hope this helps
ano ang scientific method
Answers
Answer:
The scientific method is an empirical method of acquiring knowledge that has characterized the development of science since at least the 17th century. It involves careful observation, applying rigorous skepticism about what is observed, given that cognitive assumptions can distort how one interprets the observation.
1. Consider the following mechanism. [4 Marks]
03 O2 + 0 (fast)
03+0202 (slow)
(a) Write the overall balanced chemical equation.
(b) Identify any intermediates within the mechanism.
(c) What is the order with respect to each reactant?
(d) Write the rate law for the overall reaction.
Answers
Consider the following mechanism.
The overall balanced chemical equation : 2O₃ ----> 3O₂
The intermediates within the mechanism : O
The order with respect to each reactant : 2
The rate law for the overall reaction : R = k[O₃]²/[O]
The equations are :
O₃ ----> O₂ + O fast
O₃ + O ---> 2O₃ slow
a) The overall reaction is given as :
2O₃ ----> 3O₂
b) The intermediates within the mechanism is O.
c) The order with respect to each reactant is 2
d) slow step rate : k[O][O₃]
at equilibrium, kc = [O][O₂] / [O₃]
The rate law = R = k[O₃]²/[O]
Thus, Consider the following mechanism.
The overall balanced chemical equation : 2O₃ ----> 3O₂
The intermediates within the mechanism : O
The order with respect to each reactant : 2
The rate law expression for the reaction : R = k[O₃]²/[O]
To learn more about rate law here
https://brainly.com/question/21256997
#SPJ1
which of the following options correctly describe how to calculate a molecular or formula mass for a compound? select all that apply.
Answers
Cations and anions have atomic masses that are almost identical to those of neutral elements. Ca(NO3)2 is an example of a formula. The formula/molecular mass is determined by adding the masses of the constituent elements in the right ratios.
How are the molecular mass and formula calculated?
By applying the relevant compound's chemical formula and atomic masses learned from the periodic table. The compound's formula or molecular mass is determined by adding the atomic masses in the right ratios. Which of the following statements best encapsulates the proper way to name a binary covalent compound? - With its roots and the prefix -ide, the second element is given a name.
Learn more about molecular mass from here:
https://brainly.com/question/14122402
#SPJ4
2. The chemical equation below represents the formation of hydrochloric acid.
H₂(g) + Cl₂(g) → 2 HCl(g)
If both gases are measured under STP conditions, what volume of H₂ gas will react
completely with 22.4 liters of Cl₂ gas?
Answers
One mole of H₂ reacts with one mole of Cl₂ to give 2 moles of hydrogen chloride gas. The volume of H₂ that will completely react with Cl₂ is 22.4 litres.
What is a mole?In the International System of Units, the mole is the unit of amount of a substance. It is specified that the mole contains exactly 6.022 * 10²³ elementary particles such as atoms, ions or molecules, similar to how a dozen denotes twelve.
At standard temperature and pressure (STP), one mole of any gas occupies a volume of 22.4 litres. From the given equation, the molar ratio of hydrogen and chlorine is 1:1. Since 22.4 litres of chlorine gas is used, then similarly, 22.4 litres of hydrogen gas must also be used up to give 2 moles of the product, hydrogen chloride gas.
To find out more about STP conditions, visit:
https://brainly.com/question/29129606
#SPJ1
How many moles of H2SO4 are required to completely react with 7.20 mol of Al according to the balanced chemical reaction: 2Al(s) +3H2SO4(aq) → Al2(SO4)3(aq) +3H2(g)
Answers
10.8
7.2mol*3/2=10.8mol.
10.8 moles of H₂SO₄ is required for the reaction.
The balanced equation for the reaction is given below:
2Al + 3H₂SO₄ —> Al₂(SO₄)₃ + 3H₂From the balanced equation above,
2 moles of Al required 3 moles of H₂SO₄.
Finally, we shall determine the number of mole of H₂SO₄ required to react with 7.20 moles of Al.
This can be obtained as follow:
From the balanced equation above,
2 moles of Al required 3 moles of H₂SO₄.
Therefore,
7.20 moles of Al will require = \(\frac{7.2 X 3}{2} \\\\\) = 10.8 moles of H₂SO₄
Thus, 10.8 moles of H₂SO₄ is required for the reaction.
Learn more: https://brainly.com/question/18474161
What is the lengthening of a muscle without damage? And what is the shortening of a muscle?
Answers
Answer:
Stretching is the lengthening and contraction is the shortening
Explanation:
Unless it's asking for eccentric as the stretching and concentric as the shortening
What is the calibration of this graduated cylinder? calibration
A. 5 mL
B. 2 mL
C. 1 mL
D. 10 mL

Answers
The answer is 1ml. The answer is 1ml because of calibration of this graduated cylinder
Answer:
1 mL
Explanation:
According to your definition, it is the difference between marked spaces divided by the # of spaces between marked values.
Difference between 2 marked values: 5 mL
# Of Spaces between marked values: 5
Calibration: 5 mL / 5 mL = 1 mL
The partial pressures of gases A, B, and C in a mixture are
0.75 atmosphere, 0.25 atmosphere, and 1.25 atmospheres,
respectively. What is the total pressure of the gas mixture
in millimeters of Hg?
A. 1710 mm of Hg
B.
1140 mm of Hg
C. 760.0 mm of Hg
D. 570.0 mm of Hg
Answers
Answer:
A
Explanation:
.75 + .25 + 1.25 = 2.25 atm
1 atm is 760 mm hg
2.25 * 760 = 1710 mm HG
Answer:
\(\huge\boxed{\sf 2.25\ atm = 1710\ mm\ of\ Hg}\)
Explanation:
Partial pressure of gas A = 0.75 atm
Partial pressure of gas B = 0.25 atm
Partial pressure of gas C = 1.25 atm
Total partial pressure = 0.75 atm + 0.25 atm + 1.25 atm
= 2.25 atm
We know that:
1 atm = 760 mm of Hg
Multiply 2.25 to both sides
2.25 atm = 760 × 2.25 mm of Hg
2.25 atm = 1710 mm of Hg
\(\rule[225]{225}{2}\)
The graph below shows the speed of a downhill
skier during a period of several seconds. Use the
graph to answer Question 13.
16
12
Speed (m/s)
8
0
1
3
3
2
Time (s)
13. Read Graphs What is the skier's acceleration?
Answers
Answer:
16
Explanation:
Can any help me answer this pls

Answers
Refer to the attachments
Hydrogen Fluoride-1Potassium Fluoride-2Ethane-3Ethene-4



If light with a wavelength of 515 nm is shown on a metal surface, and photoelectrons (electrons ejected from the surface) have a kinetic energy of 86.2 kJ/mol, what is the binding energy of the electrons (also known as the work function of the surface)?
Answers
The binding energy of the electrons (also known as the work function of the surface) is determined as 2.43 x 10⁻¹⁹ J.
Binding energy of the electrons
The binding energy of the electrons is also known as work function of the metal and it is calculated as follows;
Ф = E - K.E
where;
Ф = hf - 86.2 kJ/mol
Ф = hc/λ - 86.2 kJ/mol
Ф = (6.63 x 10⁻³⁴ x 3 x 10⁸ )/515 x 10⁻⁹ - 86.2 kJ/mol
Ф = 3.86 x 10⁻¹⁹ J - (86200 J/mol)/(6.02 x 10²³)
Ф = 3.86 x 10⁻¹⁹ J - 1.43 x 10⁻¹⁹ J
Ф = 2.43 x 10⁻¹⁹ J
Learn more about work function here: https://brainly.com/question/19427469
#SPJ1
Please help I only need this one to finish!

Answers
Answer:
2CO(g) + O2(g) ⇌ 2CO2(g)
Increasing the concentration of CO - ↓ ↓ ↑
Increasing the concentration of CO2 - ↑ ↑ ↓
Explanation:
The given reaction is
2CO(g) + O2(g) ⇌ 2CO2(g)
a) When the concentration of CO is increased, the stress is relieved as the reaction that consumes the added CO occurs more rapidly than its reverse reaction, for example., the forward reaction rate increases. The equilibrium will shift in favor of the product. However, the concentration of reactants (CO and O2) decreases and product concentration (CO2) increases.
b) When the concentration of CO2 is increased, the stress is relieved as the reaction that consumes the added CO2 occurs more rapidly than its reverse reaction, for example., the rate of backward reaction increases. The equilibrium will shift in favor of the reactant. Therefore, the concentration of reactants (CO and O2) increases, and the product concentration (CO2) decreases.
Hope this helps!
a sample of nitrogen gas exerted a pressure of 1 atmosphere when kept in a container of volume 300cm3 in a refrigerator at a temperature of 3C. the gas is transferred to a larger container and allowed to reach a temperature of 25C and a pressure of 0.8 atmosphere. what is the volume of the larger container
Answers
Answer:
We can use the combined gas law to solve this problem:
(P1V1/T1) = (P2V2/T2)
where:
P1 = 1 atm (pressure of nitrogen gas in the first container)
V1 = 300 cm^3 (volume of the first container)
T1 = 3°C + 273.15 = 276.15 K (temperature of the nitrogen gas in the first container, converted to Kelvin)
P2 = 0.8 atm (pressure of nitrogen gas in the second container)
V2 = ? (volume of the second container, what we want to find)
T2 = 25°C + 273.15 = 298.15 K (temperature of the nitrogen gas in the second container, converted to Kelvin)
Plugging in the values, we get:
(1 atm x 300 cm^3) / 276.15 K = (0.8 atm x V2) / 298.15 K
Simplifying and solving for V2, we get:
V2 = (1 atm x 300 cm^3 x 298.15 K) / (0.8 atm x 276.15 K)
V2 = 1309.5 cm^3
Therefore, the volume of the larger container is approximately 1309.5 cm^3.
12. What is the chemical name for the compound
CH3CH₂CH₂CH3?
(1) butane
(3) decane
(2) butene
(4) decene
Answers
The chemical name for the compound CH3CH₂CH₂CH3 is butane.
What is alkane?Alkanes are any acyclic saturated hydrocarbon with a carbon to carbon single bond e.g. methane, ethane etc.
Alkanes have a general molecular formula of CnH2n+2. The number of carbon atoms determines the name of the alkane member.
According to this question, a chemical compound with the molecular formula; CH3CH₂CH₂CH3 is given. This compound posseses 4 carbon atoms and 10 hydrogen atoms, hence, is butane.
Learn more about alkane at: https://brainly.com/question/31386716
#SPJ1
Fast, slow, why do some reactions occur faster than others?
Answers
Answer:
When the concentration of a reactant increases, there will be more chemical present. Due to more reactant particles moving together, more collisions are allowed to happen and with that, the rate of the reaction is increased. So, the higher the concentration of reactants, the faster the reaction rate will be.
Hope this helped you! :)
Heat is added to ice at 0 °C. Explain why the temperature of the ice does not change. What does change?
Answers
Heat is added to ice at 0 °C. Explain why the temperature of the ice does not change. What does change?When heat is added to ice at 0°C, the temperature of the ice does not change. This happens because all the heat energy is used up in overcoming the intermolecular forces of attraction (hydrogen bonds) that exist between the water molecules in ice.
As a result, the ice undergoes a phase change, from a solid to a liquid. This process is called melting. During melting, the temperature of the ice remains constant at 0°C because all the heat energy is used up in overcoming the intermolecular forces of attraction.The energy required to melt ice is known as the heat of fusion. The heat of fusion is the amount of heat energy required to change 1 kilogram of a solid into a liquid at its melting point. For water, the heat of fusion is 334 kJ/kg. This means that 334 kJ of heat energy is required to melt 1 kg of ice at 0°C. Therefore, during the melting of ice, the temperature of the ice does not change, but the internal energy of the ice does change, and this is manifested in the change of phase from a solid to a liquid.In summary, when heat is added to ice at 0°C, the temperature of the ice does not change, and all the heat energy is used up in overcoming the intermolecular forces of attraction between the water molecules in ice. This results in the melting of ice without any change in temperature.For such more question on molecules
https://brainly.com/question/475709
#SPJ8
Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory to standardize strong base solutions. It has the unwieldy formula of KHC8H4O4. This is often written in shorthand notation as KHP. Of 5.06 grams of KHP are needed to exactly neutralize 35.3 mL of a barium hydroxide solution, what is the concentration of the base solution
Answers
Question: Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory to standardize strong base solutions. It has the unwieldy formula of KHC8H4O4. This is often written in shorthand notation as KHP. Of 5.06 grams of KHP are needed to exactly neutralize 35.3 mL of a barium hydroxide solution, what is the concentration of the base solution?
Explanation and answer:
2KHP + Ba(OH)2 ---> 2H2O + Ba(KP)2
you need 2 moles of KHP to neutralise 1 mole of Ba(OH)2
moles KHO = mass / molar mass
= 2.43 g / 204.22 g/mol
= 0.0118989 mol
moles Ba(OH)2 = 1/2 x moles KHP
= 0.005949 mol
Molarity Ba(OH)2 = moles / Litres
= 0.005949 mol / 0.0213 L
= 0.279 M (3 sig figs)
Define or give an example for each of the following terms.
a. hydrocarbon
b. alkyne
c. alkane
d. alkene
Answers
a) A combound which contains only Carbon and Hydrogen. There are covalent bonds between atoms. Hydrogen form one single bond and Carbon forms four covalent bonds. Carbon bonds can be single, double or triple bonds.
All hydrocarbons are organic compounds, but organic compound can include atoms of other elements.
b) Alkyne has a covalent triple bond between two carbon atoms. Simplest alkyne is ethyne HCCH.
b) Alkane contains only Carbon and Hydrogen and there are single bonds
between atoms. Simplest alkane is methane CH4.
c) An alkene has one double bond between Carbon atoms. Simplest
alkene is ethene H2C=CH2.
What type of reproduction occurs in members of the bacteria kingdom?
Answers
Answer:
Bacteria can use sexual and asexual repoduction
Explanation:
A compound of P and F was analyzed as follows: heating 0.2324 g of the compound in a 378-cm3 container turned all of it to gas, which had a pressure of 97.3 mmHg at 77°C. Then the gas was mixed with calcium chloride solution, which turned all of the F to 0.2631 g of CaF2. Determine the molecular formula of the compound.
Answers
The molecular formula of the compound is determined as P₂F₄.
What is meant by molecular formula?Molecular formula tells us which atoms and how many of each type of atom are present in the molecule.
Molar mass of CaF₂ is 78.07 g/mol(0.2631 g CaF₂) × (1 mol CaF₂ / 78.07 g CaF₂) = 0.00337 mol CaF₂
2F- + Ca₂+ → CaF₂
(0.00337 mol CaF₂) × (2 mol F / 1 mol CaF₂) = 0.00674 mol F
As, Molar mass of F is 18.9984 g/mol
So, (0.00674 mol F) × (18.9984 g F / 1 mol F) = 0.12805 g F
1 mmHg = 0.00131578947 atm
(97.3 mmHg) × (0.00131578947 atm / 1 atm) = 0.128 atm
(378 cm³) × (1L / 1000 cm³) = 0.378 L
Now, T = 77 + 273 = 350 K
As, PV = nRT
n(PxFy) = PV / RT
n(PxFy) = (0.128 atm × 0.378 L) / (0.0821 L atm mol-1 K-1 × 350 K) = 0.001684 mol
n(PxFy) = 0.001684 mol
n(PxFy) = m(PxFy) / M(PxFy)
M(PxFy) = m(PxFy) / n(PxFy) = (0.2324 g) / (0.001684 mol) = 138 g/mol
m(PxFy) = m(P) + m(F)
0.2324 g = m(P) + 0.12805 g
m(P) = 0.2324 - 0.12805 = 0.10435
m(P) = 0.10435 g
Molar mass of P is 30.9737 g/mol
so, (0.10435 g P) × (1 mol P / 30.9737 g P) = 0.003369 mol P
Now, n(P) : n(F) = 0.003369 mol : 0.00674 mol = 1 : 2
Therefore, empirical formula of PxFy is PF₂
Molar mass of PF₂ 68.9705 g/mol
Molecular formula of PxFy is ( PF₂)n
n = M(PxFy) / M( PF₂) = (138) / (68.9705) = 2
Therefore, the molecular formula of PxFy is P₂F₄.
To know more about molecular formula, refer
https://brainly.com/question/13058832
#SPJ1
PLEASE HELP IMMEDIATELY I NEED THE ANSWER NOT A HINT THANK YOU
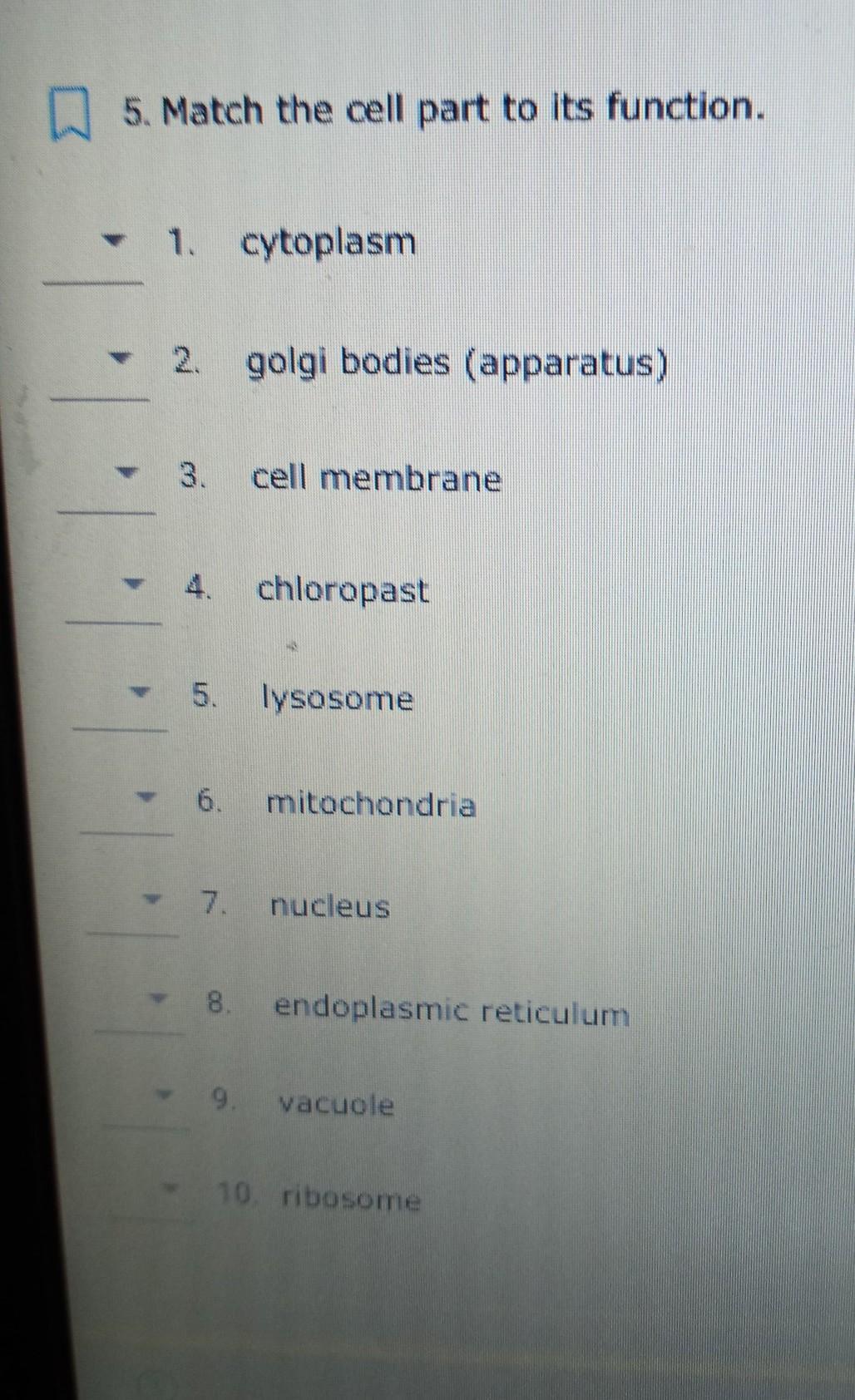
Answers
Cytoplasm: gel like environment which allows organelles to move about the cell
Golgi bodies: packages and ships materials out of the cell
Cell membrane: controls what goes in and out of the cell
Chloroplast: makes food for plant cells using sunlight
Lysosome: breaks down waste, food, and worn out cell parts
Mitochondria: breaks down food to release energy for the cell
Nucleus: contains the cell's DNA and is the control center of the cell
Endoplasmic reticulum: transports materials within cell; process lipids
Vacuole: stores water, waste and food
Ribosome: make proteins
if a gas at 25.0 °C occupies 3.60 liters at a pressure of 104.3kPa what will be its volume at a pressure of 2.50atm
Answers
From the question, we will need to assume the temperature don't change.
In this case, assuming the gas behaves as an ideal gas, we can use the Boyle's Law to answer.
The Boyle's Law says that the product of the pressure and volume of an ideal gas, at constant temperature, is a constant:
\(pV=k\)But the volume and pressure have to be on the same unit.
First, lets convert the final pressure of 2.50 atm to kPa. We can do that by multiplying the atm value by 101.325:
\(p_2=2.50atm=2.50\cdot101.325kPa=253.3125kPa\)Now, we can apply the Boyle's Law:
\(\begin{gathered} p_1V_1=k \\ p_2V_2=k \\ p_2V_2=p_1V_1_{} \\ V_2=\frac{p_1V_1}{p_2_{}}=\frac{104.3kPa\cdot3.60L}{253.3125kPa}=1.4822\ldots L\approx1.48L_{} \end{gathered}\)So, its volume will be approximately 1.48 L.
how would you confirm the presence of lead in an ore?
Answers
There are numerous ways to determine whether lead is present in an ore. Atomic absorption spectroscopy is a popular approach. With this method, an ore sample is dissolved in acid and then atomized in a flame or plasma.
The sample's atoms will absorb light at particular wavelengths that are peculiar to the element under investigation. The amount of light absorbed can be used to calculate how much lead is present in the sample. Inductively coupled plasma mass spectrometry and X-ray fluorescence spectroscopy are further techniques. It is crucial to remember that these procedures call for specialized tools and training, thus they ought to only be carried out in a lab by qualified experts.
To know more about spectrometry, here
brainly.com/question/31075363
#SPJ1
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Which natural disaster is heavy rainfall most likely to cause?
earthquakes
hurricane
flooding
drought
Answers
Answer:
flooding
Explanation:
since heavy rainfall, if its greater than usual
this causes flooding
hope this helps:)
Mg(s) + Ni2+(ag) -> Mg2+ (aq) + Ni(s) What is the total number of moles of electrons lost by Mg(s) when 2.0 moles of electrons are gained by Ni2+(ag)? * 10 ( 1.0 mol ,20 mol ,3.0 mol, 4.0 mol
Answers
The total number of moles of electrons lost by Mg(s) when 2.0 moles of electrons are gained by Ni2+(ag) is also 2.0 moles of electrons.
How to find the number of moles?This is because in a chemical equation, the number of moles of electrons gained by the reducing agent (in this case Ni2+) is equal to the number of moles of electrons lost by the oxidizing agent (in this case Mg(s)).
In this redox reaction, Mg is being oxidized because it loses electrons and Ni is being reduced because it gains electrons. The oxidation and reduction process are occurring simultaneously, so the number of electrons lost by Mg(s) is equal to the number of electrons gained by Ni2+(ag).
Learn more about moles of electrons in brainly.com/question/512038
#SPJ1
The electrons that are gained by the \(Ni^{2+}\) ion is 2.0 moles of electrons.
What is the number of the electrons gained?We know that when there is a redox reaction, there would be the loss or gain of electrons in the process. The process is a simultaneous one so the electrons that are lost by one specie must as a matter of necessity be gained by another specie.
In this case, as we look at the reaction equation we can see that there are two electrons that have been lost by the magnesium atom and these two electrons would be gained by the Nickel II ion.
Learn more about redox reaction:https://brainly.com/question/13293425
#SPJ1