enter a balanced chemical equation for the fermentation of glucose (c6h12o6) by clostridium pasteurianum in which the aqueous sugar reacts with water to form aqueous acetic acid (ch3co2h) , carbonic acid (h2co3) , and hydrogen gas. express your answer as a chemical equation including phases.
Answers
The balanced chemical equation for the fermentation of glucose, we can see that methanol and carbon dioxide are formed.
Fermentation, is generally a chemical process by which molecules such as glucose are broken down anaerobically or in the absence of water. More broadly we can understand that fermentation is the foaming that occurs during the manufacture of wine and beer, a process at least 10,000 years old.
Fermentation occurs in yeast cells, and a form of fermentation takes place in bacteria and in the muscle cells of the animals. In yeast cells these are those yeast used for baking bread and also that are producing alcoholic beverages, glucose can be metabolized through cellular respiration as in other cells too.
The balanced chemical equation for the fermentation of glucose is as follows;
\(C_{12}H_{22}O_{11}\) + \(H_{2}O\) → \(4C_{2}H_{5}OH\) + \(4CO_{2}\)
To know more about balanced chemical equation
https://brainly.com/question/28294176
#SPJ4
Related Questions
1. Name the elements with the following symbols
a. Na
b. H
c. C
d. O
2. What is the symbol for the following elements?
a. Helium
b. Sulfur
c. Copper
d. Lead
Answers
Answer:
1.
a. Sodium
b.Hydrogen
c Carbon
d Oxygen
2.
a He
b S
c Cu
d Pb
Answer:
1.
a. Sodium
b.Hydrogen
c Carbon
d Oxygen
2.
a He
b S
c Cu
d Pb
Explanation:
Hope this will help
What ideas do you have about what makes Anchorage, Alaska, cooler than Christchurch, New Zealand?
-science
Answers
Answer:
You can explore a variety of things in Alaska. There are so many activities you could do such as visiting museums and hiking and tons more. The thing about this is that it is out in the wilderness which makes it more exciting and cooler to see nature more than it does in new zealand. The climate can be pretty chilly in Alaska but it is way much fun to see tons of glaciers. New zealand has eathquakes frequently whilst Alaska doesn't.
125 ml of nitrogen gas is collected at 70.0 degrees Celsius. The pressure
in the container is 125 kPa. What will be the volume of the gas at STP?
Round and record your answer exactly like the given volume of 125 ml (3
digits) with a space then the unit.
PLS HELP!! due at the end of the day :(
Answers
Answer: Volume of the gas at STP is 22.53 L.
Explanation:
Given : Volume = 125 mL (as 1 mL = 0.001 L) = 0.125 L
Temperature = \(70^{o}C = (70 + 273) K = 343 K\)
Pressure = \(125 kPa = 125 kPa \times \frac{0.01 atm}{1 kPa} = 1.25 atm\)
According to the ideal gas equation, the volume of given nitrogen gas is calculated as follows.
PV = nRT
where,
P = pressure
V = volume
n = number of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
\(1.25 atm \times V = 1 mol \times 0.0821 L atm/mol K \times 343 K\\V = \frac{1 mol \times 0.0821 L atm/mol K \times 343 K}{1.25 atm}\\= \frac{28.1603}{1.25} L\\= 22.53 L\)
Hence, volume of the gas at STP is 22.53 L.
Elements that are in the same _____ have the same number of electrons in their outer ______. These outer electrons are so important in determining the chemical ______ of an element that a special way to represent them has been developed. A(n) _____ uses the symbol of the element and dots to represent the electrons in the outer _______
Answers
Elements that are in the same group have the same number of electrons in their outer shell. These outer electrons are so important in determining the chemical properties of an element that a special way to represent them has been developed. A(n) electron dot diagram uses the symbol of the element and dots to represent the electrons in the outer shell.
The elements in the same group have the same number of electrons in their outer shell because they have the same number of valence electrons. These valence electrons are responsible for the chemical properties of an element, which is why elements in the same group have similar chemical properties. The symbol of the element is used in the electron dot diagram to represent the element, and the dots are used to represent the valence electrons in the outer shell. This diagram is a useful tool for understanding the chemical behavior of an element and for predicting how it will react with other elements.
You can learn more properties of elements at: brainly.com/question/30388913
#SPJ11
Fe(s)+2HBr(aq)→FeBr2(aq)+H2(g)Fe(s)+2HBr(aq)→FeBr2(aq)+H2(g) Part A What mass of HBrHBr (in gg) would you need to dissolve a 3.2-gg pure iron bar on a padlock?
Answers
The mass of HBr (in g) you would need to dissolve a 3.2-gg pure iron bar on a padlock is 9.27 g.
The given chemical equation is:
Fe(s) + 2HBr(aq) → FeBr₂(aq) + H₂(g)
The balanced chemical equation indicates that 1 mole of Fe reacts with 2 moles of HBr and produces 1 mole of H₂ and 1 mole of FeBr₂. We are to determine the mass of HBr required to dissolve a 3.2 g pure iron bar on a padlock.
First, find the molar mass of Fe. To find the molar mass of Fe, we need to find its atomic mass from the periodic table.
Atomic mass of Fe = 55.85 g/mol
Molar mass of Fe = 55.85 g/mol
Find the number of moles of Fe present in a 3.2 g sample using the following formula:
Number of moles = Mass/Molar mass
Number of moles of Fe = 3.2 g/55.85 g/mol = 0.057 mol
Find the number of moles of HBr required for the reaction. To dissolve 0.057 mol of Fe, we need 0.057 mol x 2 mol HBr/1 mol Fe = 0.114 mol of HBr
Find the mass of HBr required. To find the mass of HBr required, we can use the following formula:
Mass = Number of moles x Molar mass
Mass of HBr required = 0.114 mol x 80.91 g/mol = 9.27 g
Therefore, the mass of HBr required to dissolve a 3.2-g pure iron bar on a padlock is 9.27 g.
Learn more about balanced chemical equation here: https://brainly.com/question/26694427
#SPJ11
which phenomenon that goes unexplained by lewis structures is solved by applying molecular orbital theory?
a. bond angles
b. ionization energy trends
c. none of the above
Answers
If you use the molecular orbital theory, option C, which is the paramagnetism of oxygen particles, you can explain the odd thing that Lewis structures can't explain.
The fact that oxygen particles are paramagnetic doesn't make sense in terms of Lewis structure. Since oxygen atoms have two electrons that don't have a partner, only molecular orbital theory can explain why oxygen particles are paramagnets. A Lewis structure is a much better way to show how the electrons in a particle's valence shell are arranged. It is used to show how the electrons around the different atoms in a particle are arranged. Electrons are shown as "dabs" or as a line running between two particles. In his cubical particle hypothesis, Lewis came up with the "octet rule." The octet rule is based on the fact that iotas tend to like having eight electrons in their valence shell. When molecules have fewer than eight electrons, they usually react by making more stable mixtures. Atoms will act to get into the most stable state possible.
To know more about paramagnetism click on the link below:
https://brainly.com/question/2272751
#SPJ4
More people in Australia have skin that is easily damaged by sunlight?
Answers
an aqueous sodium nitrate (nano3) solution must be created for an experiment. if 50.00 ml of a 0.150 m solution is needed, what amount of sodium nitrate (in grams) must be weighed out?
Answers
If you need to make a na2so4 nitrate solution for an experiment, weigh out 0.6375 g of sodium nitrate.
Experiment? What do you mean?a method used in a controlled setting to find a previously undiscovered effect or law, validate a theory, or provide an example of an existing law. experimenting, the act of testing
What was the outcome of the experiment?An experiment is a procedure used to verify or refute a theory, as well as gauge the success or viability of something that hasn't been done before. Experiments illuminate cause-and-effect linkages by demonstrating how happens when a specific ingredient is changed.
To know more about experiment visit:
https://brainly.com/question/30055326
#SPJ4
Did the pcr reagents and thermal cycler function as expected? what evidence do you have for this conclusion?
Answers
The assessment of PCR reagents and thermal cyclers performance depends on the specific experiment and the desired outcome.
the performance of PCR reagents and thermal cyclers can be evaluated based on various factors. Here are some indicators that can be used to assess their performance: Amplification Efficiency: PCR reagents should efficiently amplify the target DNA or RNA sequences. This can be determined by analyzing the amplification curves generated during the PCR process. A sharp and distinct amplification curve with appropriate amplification levels indicates the successful function of PCR reagents and thermal cyclers. Specificity: PCR reagents should specifically amplify the target sequence without generating non-specific products.
Learn more about thermal cyclers here:
https://brainly.com/question/31182664
#SPJ11
Which two features of Earth's crust involve tension?
O A. Convergent boundaries
B. Reverse faults
c. Divergent boundaries
O D. Normal faults
Answers
Answer:
The correct options are;
C. Divergent plate boundary
D. Normal faults
Explanation:
Tensile stress tends to pull objects part by acting axially upon the object to pull the object on a cross section perpendicular to the objects cross-section
The most common stress in convergent plate boundaries is compression stress
The most common stress in divergent plate boundaries is tensile stress
In the presence of tensile stress, normal faults results in the raising of mountains due to their enormous forces
Therefore, the features of Earth's crust involving tension are divergent plate boundary and normal faults.
Answer:c and d
Explanation:
1.4 Name three properties of discrete Fourier transform (DFT)
Answers
To summarize, the three properties of DFT include periodicity, time-shift, and linearity. The DFT is an important mathematical tool for signal processing, and it is utilized to transform a discrete-time sequence from the time domain to the frequency domain.
The three properties of Discrete Fourier Transform (DFT) are as follows:Periodicity: The discrete Fourier transform has a periodicity that is equal to the length of the data sequence. For example, if the DFT of a sequence of N points is performed, the resulting transform will repeat itself after N points of frequency or spectral information have been computed.Time-shift: The discrete Fourier transform is sensitive to the time shift of a sequence. For instance, the DFT of a time-shifted signal is a complex exponential multiplied by the DFT of the original sequence.Linearity: The discrete Fourier transform satisfies the principle of superposition. It implies that if two separate inputs x(n) and y(n) are given, then the transform of the sum of these two inputs is equal to the sum of the transform of the two inputs.To summarize, the three properties of DFT include periodicity, time-shift, and linearity. The DFT is an important mathematical tool for signal processing, and it is utilized to transform a discrete-time sequence from the time domain to the frequency domain.
To know more about processing visit:
https://brainly.com/question/31815033
#SPJ11
Please help me with this

Answers
According to its nutrition label, orange soda contains 49 g of sugar per 355-mL serving. If the density of the beverage is 1.043 g/mL, what is the percent sugar concentration in orange soda? (Hint: This is a two-step problem. First use the density to convert the 355-mL serving size to grams. Then calculate percent sugar in the beverage.)
Answers
The percent sugar concentration in orange soda is approximately 13.24%.
The volume of orange soda is given as 355 mL and its density is given as 1.043 g/mL. According to the nutrition label, there are 49 g of sugar in a 355 mL serving of orange soda.Using the density, we can convert the 355 mL volume into grams as follows:Volume = 355 mL; Density = 1.043 g/mL; Mass = ?To convert mL to g we need to multiply the volume with the density. Thus,Mass = Volume x Density= 355 x 1.043= 369.965 gThus, the mass of a 355 mL serving of orange soda is approximately 369.965 g.Next, we can calculate the percentage of sugar in the beverage as follows:Percent sugar concentration = (Mass of sugar / Total mass of beverage) x 100%Percent sugar concentration = (49 g / 369.965 g) x 100%Percent sugar concentration = 0.1324 x 100%Percent sugar concentration = 13.24%Therefore, the percent sugar concentration in orange soda is approximately 13.24%.
learn more about concentration
https://brainly.com/question/15306782
#SPJ11
what are buffers in chemistry
Answers
A buffer is a chemical solution that can withstand pH fluctuations when an acid or base is added to it. A weak acid and its conjugate base, or a weak base and its conjugate acid, make up a buffer system. A solution
A buffer is a chemical solution that can withstand pH fluctuations when an acid or base is added to it. Weak bases and their conjugate bases, or weak acids and their conjugate bases, make up buffers. The weak acid or base exists in the buffer system in balance with its conjugate base or acid, and this equilibrium aids in the buffer's ability to keep a constant pH level. In many chemical, biological, and industrial applications where pH regulation is crucial, buffers are useful. For instance, the pH of the blood in the human body is kept within a certain range by a buffer system, and many laboratory investigations call for the use of buffers to buffer
Learn more about buffer here:
https://brainly.com/question/22821585
#SPJ4
Is a covalent bond formed between a metal and a non-metal?
Answers
No, Covalent bond cant not be formed between a metal and non-metal. ionic bonds between metals and nonmetals.
A covalent bond is formed when two atoms exchange one or more pairs of electrons. The two atomic nuclei are concurrently drawing these electrons to them. When the difference between the electronegativities of two atoms is too tiny for an electron transfer to take place to create ions, a covalent bond is formed. Bonding electrons are collectively referred to as the electrons that are present between the two nuclei. The "glue" that holds the atoms in molecular units together is the bound pair. By taking into account whether each element is a metal or nonmetal, it is possible to forecast the kind of bond that will develop between two elements. Covalent bonds between nonmetals, ionic bonds between metals and nonmetals, and metallic bonds between metals are the three types of bonds that typically form. Covalent bonds, which produce an irreversible binding and a high surface coverage, are mostly generated between side-chain-exposed functional groups of proteins and properly modified transducer surfaces. Covalent immobilisation is one of the most often utilised techniques, and it involves randomly coupling the antibody's free amino groups to a sensor surface.
For much more questions on covalent bond, Refer:
https://brainly.com/question/12732708
#SPJ4
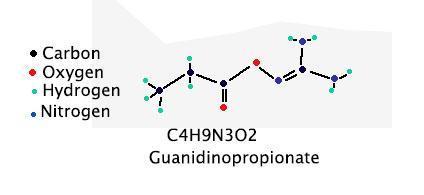
does someone know how to draw a structural formula of H1O2N3C4 please
Answers
Answer:
This could be guanidinopropionate. A drawing of the structure is attached.
Explanation:
There are other possible structures with this formula. The one shown in the attached diagram is one possibility. It is named, unfortunately, guanidinopropionate.
explain your answer with given reason
warning:(if you get reported more then 5 times. then you will lose your account

Answers
Im not really sure but my best guess is d 52.8%
in order tp inflate a cars airbags to the proper size at standard temperature and pressure, 67 l of n2 gas are needed. Hpw many moles of n2 gas is this
Answers
67 L of N2 gas at STP is equivalent to 2.67 moles of N2 ga
Steps
To calculate the number of moles of N2 gas, we can use the ideal gas law:
PV = nRT
where:
P is the pressure of the gas
V is the volume of the gas
n is the number of moles of gas
R is the universal gas constant
T is the temperature of the gas in kelvin
Assuming standard temperature and pressure (STP), the pressure is 1 atmosphere and the temperature is 273 K. The volume of gas is 67 L.
So, we have:
PV = nRT
n = (PV) / (RT)
n = (1 atm) x (67 L) / ((0.0821 L·atm/mol·K) x (273 K))
n = 2.67 mol
Therefore, 67 L of N2 gas at STP is equivalent to 2.67 moles of N2 gas.
Gases in a Salt Marsh You measure appreciable CH4(g) and CO2 g) partial pressures in the sediment pore fluid and you want to know if there is equilibrium between these redox couples. The measured partial pressure of CO2(g) is 1.10 atm and the measured partial pressure of CH(g) is 5.10 atm in the pore fluid. You measure a redox potential (Eh) of pore-fluid water of El -0.1 V at pH 8.3 h using a platinum electrode and suitable reference electrode. Start by writing a balanced reduction reaction where CO2(g) is converted into CH4(g This is a calculation that employs the attached thermodynamic data. a) What ratio of partial pressures of CO2(g) and CH4(g) to you expect if the the Eh were a reliable guide to the equilibrium state of these two gases? b) Calculate a value of Eh from the ratio of the measured gas partial pressures. his will not agree with the previous answer.)
Answers
a) The expected ratio of partial pressures of CO₂(g) and CH₄(g) is 1.75 x 10^14.
b) The value of Eh from the ratio of the measured gas partial pressures is 0.27 V.
The balanced reduction reaction for the conversion of CO₂(g) to CH₄(g) is:
CO₂(g) + 4H⁺ + 4e⁻ -> CH₄(g) + 2H₂O
a) Using the thermodynamic data, we can calculate the equilibrium constant (K) for this reaction at the given conditions (pH 8.3 and El -0.1 V). From the table, we can see that ΔG° = -162.9 kJ/mol. Using the equation ΔG = -RTlnK, we can calculate K to be 1.76 x 10^14. This means that the ratio of the partial pressures of CH₄(g) to CO₂(g) at equilibrium is:
K = [CH₄(g)] / [CO₂(g)]
1.76 x 10^14 = [CH₄(g)] / 1.10 atm
[CH₄(g)] = 1.93 x 10^14 atm
Therefore, the expected ratio of partial pressures is:
[CH₄(g)] / [CO₂(g)] = 1.93 x 10^14 atm / 1.10 atm = 1.75 x 10^14
b) To calculate the Eh from the measured partial pressures of CO₂(g) and CH₄(g), we can use the Nernst equation:
Eh = E° + (RT/nF)ln(Q)
Where E° is the standard reduction potential (0.21 V for the reaction CO₂(g) + 4H⁺ + 4e⁻ -> CH₄(g) + 2H₂O), R is the gas constant, T is the temperature (assumed to be 25°C), n is the number of electrons transferred in the reaction (4), F is the Faraday constant, and Q is the reaction quotient.
Q = [CH₄(g)] / [CO₂(g)]
Q = 5.10 atm / 1.10 atm
Q = 4.64
Plugging in the values, we get:
Eh = 0.21 V + (0.0257 V/n) ln(4.64)
Eh = 0.21 V + 0.057 V
Eh = 0.27 V
Therefore, the calculated value of Eh from the measured gas partial pressures does not agree with the value of El -0.1 V measured using the platinum electrode and reference electrode. This suggests that the redox couples of CO₂(g) and CH₄(g) are not at equilibrium, and other factors may be influencing their partial pressures in the sediment pore fluid.
Learn more about partial pressures at https://brainly.com/question/31196860
#SPJ11
Bar Magnet Number
(B)
Location of Magnet
(Find 3 separate
locations where the
magnet number is
high, medium and low)
Bar Magnet
G
Magnetic (B) Field
B
5.26 G
Bx
3.20 G
G
Ву
4.17 G
52.52 0

Answers
Answer:
tell me how you want
this done?
Explanation:
Engineers wanted to redesign a mouse that was more reliable and that _____
Answers
Engineers wanted to redesign a mouse that was more reliable and that was cheaper.
The engineers understood that in order to make the mouse significantly less expensive and more dependable, they would need to drastically simplify its fundamental mechanical design, employ more durable but less expensive materials, and streamline manufacturing.
The mouse developed by Xerox PARC was an expensive, fragile, and failure-prone work of high-concept technology that had no chance of being successful as a commercial product. The state of the art in plastic moulding was pushed due to the size of some of the parts and the tolerances required in the final design. A skilled manufacturer might produce plastic with tolerances of a thousandth of an inch for a reasonable price.
To know more about the mouse, refer:
https://brainly.com/question/3280371
#SPJ4
Can someone bold enough post a video about the in's and out's of molarity (Practice problems and all) and post the link to in the answer column!
If their is no video, or the video is off topic upon review, I will have to contact the staff at brainly.
Answers
There are several videos available that cover the in's and out's of molarity, including practice problems. One of the best sources for such videos is the Khan Academy, which offers a wide range of educational videos on topics such as chemistry, math, biology, physics, and more. Their videos are high-quality, comprehensive, and easy to follow, making them ideal for students who want to learn more about molarity and other related concepts in chemistry.
Yes, there are several videos available that cover the in's and out's of molarity, including practice problems. One of the best sources for such videos is the Khan Academy, which offers a wide range of educational videos on topics such as chemistry, math, biology, physics, and more. Their videos are high-quality, comprehensive, and easy to follow, making them ideal for students who want to learn more about molarity and other related concepts in chemistry.
To find a video about molarity on the Khan Academy, you can go to their website and search for the term "molarity" in the search bar at the top of the page. This will bring up a list of videos related to this topic, including some that cover the basics of molarity, while others provide more advanced information.
Once you find a video that you like, you can watch it online or download it to your computer for later viewing. Some of the most popular videos on this topic include "Molarity, molality and normality" and "Solving Molarity Problems." These videos cover a wide range of topics related to molarity, including how to calculate molarity, how to convert between different units of concentration, and how to use molarity to solve problems in chemistry.
for more questions on molarity
https://brainly.com/question/30404105
#SPJ8
What is the chemical formula for the most common fragment of formic acid?.
Answers
what types of energy are involved in a chemical reaction? activation energy is the energy required for a chemical reaction to take place, and chemical energy is the energy associated with every substance.
Answers
In a chemical reaction, there are typically two types of energy involved: activation energy and chemical energy. Activation energy is the minimum amount of energy required for a chemical reaction to occur.
This energy is needed to break the bonds between atoms or molecules in the reactants, allowing them to rearrange and form new bonds in the products. Chemical energy, on the other hand, is the energy stored within the bonds of the reactants and products themselves. It is the energy that is released or absorbed during a reaction, and it is what ultimately determines whether a reaction is exothermic (releasing energy) or endothermic (absorbing energy).
The types of energy involved in a chemical reaction are activation energy and chemical energy. Activation energy is the energy required for a chemical reaction to take place, and chemical energy is the energy associated with every substance. In a chemical reaction, the activation energy is needed to break bonds in the reactants, while the chemical energy is stored in the bonds of the reactants and products, which can be released or absorbed during the reaction.tt
To know more about chemical reaction visit:
https://brainly.com/question/29762834
#SPJ11
What are sum things yhu can do to prevent brain damamge
Answers
Answer:
not bang your head
Explanation:
Answer:
focus on your surroundings
g a flask contains a mixture of 0.67 mol he and 4.46 mol ne gases. determine the partial pressure of ne (in mm) if the total pressure is 296 mm.
Answers
Partial pressure equals total pressure minus the mole fraction. It demonstrates how a component's mole fraction and partial pressure are related.
The partial pressure of Ne is what?Neon has a partial pressure of 0.4 atm, while krypton has a partial pressure of 1.20 atm. The mole fraction has no units. He and Ne gases together have a total pressure of 1.
If the overall pressure is 760 mmHg, what is the partial pressure of argon?7.6 mmHg As a result, it can be calculated that the partial pressures of the various gases at sea level, where the atmospheric pressure is 760 mmHg, are roughly 593 mmHg for nitrogen, 160 mmHg for oxygen, and 7.6 mmHg for argon.
To learn more about atmospheric pressure here:
https://brainly.com/question/13505790
#SPJ4
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
True or False: Molar ratios come from the molar coefficients of balanced chemical reactions
True or False: The process of dimensional analysis requires the use of conversion factors *
True or False: The molar mass comes from balanced chemical reactions
Answers
Answer:
1. true
2.true
3.fasle
Explanation:
sorry if im wrong.
have a lovely day.
calculate the value of kc from the following equilibrium concentrations: [fencs2 ] = 1.84 × 10−4 m, [fe3 ] = 1.32 × 10−3 m, and [scn–] = 1.02 × 10−3 m
Answers
The equilibrium constant (Kc) is 1.37 * 10² M⁻¹.
First, we write the reaction equation:
Fe³⁺ + SCN⁻ ↔ [FeSCN]²⁺
From this, we can see that the iron(III) cation (Fe³⁺) and the thiocyanate anion (SCN⁻) are the reactants, while the iron(III) thiocyanate ion ([FeSCN]²⁺) is the product. Based on this, we can write the expression for the equilibrium constant (Kc):
\(Kc = \frac{[[FeSCN]^{2+}]}{[Fe^{3+} ][SCN^{-} ] }\)
[Fe³⁺] - equilibrium concentration of the iron(III) ion (1.32 * 10⁻³M)
[SCN⁻] - equilibrium concentration of the thiocyanate ion (1.02 * 10⁻³ M)
[[FeSCN]²⁺] - equilibrium concentration of the iron(III) thiocyanate ion (1.84 * 10⁻⁴ M)
Now we plug the known values into the expression:
Kc = 1.84 * 10⁻⁴ M / (1.32 * 10⁻³ M * 1.02 * 10⁻³M)
Kc = 1.37 * 10² M⁻¹
You can learn more about equilibrium constants here:
brainly.com/question/15118952
#SPJ4
The balanced chemical equation for the reaction between sodium chloride and silver nitrate is: NaCl(aq) AgNO3(aq) AgCl(s) NaNO3(aq) We can interpret this to mean: ... 1 mole of sodium chloride and moles of silver nitrate React to produce ... moles of silver chloride and moles of sodium nitrate
Answers
Answer:
1 mole of NaCl reacts with 1 mole of AgNO₃ to produce 1 mole of AgCl and 1 mole of NaNO₃
Explanation:
The given reaction is a double decomposition (metathesis) reaction. A reaction in which the products are formed by the exchange of the ions present in the two reactants. NaCL and AgNO₃ exchange ions to form AgCl, which precipitates and NaNO₃.
The balanced equation for reaction is given below;
NaCl(aq) + AgNO₃(aq) ----> AgCl(s) + NaNO₃(aq)
In the reaction above, the mole ratio of the reactants to products is 1 : 1 ---> 1 : 1
This means that 1 mole of NaCl reacts with 1 mole of AgNO₃ to produce 1 mole of AgCl and 1 mole of NaNO₃.
Given the molar mass of the compounds above;
NaCl = 58.5 g/mol; AgNO₃ = 170 g/mol; AgCl = 143.5 g/mol; NaNO₃ = 85 g/mol
Therefore, 58.5 g of NaCl reacts with 170 g of AgNO₃ to produce 143.5 g of AgCl and 85 g of NaNO₃