Answers
This claim is untrue. A developmental mismatch between the maturation of various brain systems, particularly the prefrontal cortex, and the limbic system, is a hallmark of the teenage brain.
The limbic system, which is engaged in emotion regulation and reward processing, develops earlier than the prefrontal cortex, which is in charge of impulse control, decision-making, and other executive processes.
Teenagers may therefore be more likely to participate in a dangerous activity and seek out unique experiences, but they may also have trouble controlling their impulses and making reasoned decisions.
The development of the brain is a complicated and ongoing process, and individual variations in neural maturation and life events can also have an impact on teenage behavior and decision-making.
However, there is no typical pattern of adolescent behavior or brain function due to individual variances and ongoing brain development.
learn more about controlling behavior here
https://brainly.com/question/6646156
#SPJ1
Related Questions
the plastic sphere is placed in 500 mL of water. The plastic sphere’s temperature is 85 °C. The water’s temperature is 15 °C. In wich direction will the thermal energy move, from the plastic to the water or the water to the plastic. Explain your answer
Answers
Answer:
Heat energy will flow from the plastic sphere to the water. This is because heat energy always flows from a warmer object to a cooler object.
Explanation:
In this case, the plastic sphere has a higher temperature of 85 °C, while the water has a lower temperature of 15 °C. Therefore, heat energy will naturally flow from the plastic sphere to the water until both objects reach thermal equilibrium, where they have the same temperature. As a result, the plastic sphere will cool down and the water will warm up until they reach the same temperature.
3: Given 12.3 grams of NH3, how many moles of N₂ were needed?
Answers
0.361 moles of N₂ were required to produce 12.3 g of NH₃, using the balanced chemical equation N₂ + 3H₂ → 2NH₃.
The balanced chemical equation for the reaction is N₂ + 3H₂ → 2NH₃. We can use the balanced equation and the molar mass of NH₃ to calculate the number of moles of N₂ required to produce 12.3 g of NH₃,
1 mol NH₃ = 2 mol N₂ (from the balanced equation)
molar mass of NH₃ = 17.03 g/mol
moles of NH₃ = 12.3 g / 17.03 g/mol
moles of NH₃ = 0.722 mol
moles of N₂ = (0.722 mol NH₃) / 2
moles of N₂ = 0.361 mol
Therefore, 0.361 moles of N₂ were needed to produce 12.3 grams of NH₃.
To know more about moles, visit,
https://brainly.com/question/29367909
#SPJ1
Complete question - For the reaction, N₂ + 3H₂ → 2NH₃. Given 12.3 grams of NH3, how many moles of N₂ were needed?
BRAINLIEST TO CORRECT what would the cook time for 2 half-lives of the popcorn?
Answers
Answer:
Pop your popcorn for 2 minutes in the microwave but if you like to pop your popcorn old fashion get popcorn seeds and put them in an pot and then put a lid over it then pop it.
Explanation:
Question:
Many island chains were formed as a result of blank volcanism
Answers
Many island chains were formed as a result of blank volcanism is known as hotspot volcanism.
Hotspot volcanism occurs when a mantle plume, a column of hot and buoyant rock material rising from deep within the Earth's mantle, reaches the surface. These mantle plumes are stationary relative to the moving tectonic plates on the Earth's surface.
As the tectonic plate moves over the stationary hotspot, the mantle plume melts and produces magma. This magma rises through the Earth's crust, creating a volcanic eruption. Over time, repeated eruptions build up layers of lava and volcanic material, forming a cone-shaped volcano. As the tectonic plate continues to move, the volcano becomes inactive, and a new volcano forms above the stationary hotspot.
However, in the case of island chains, the tectonic plate movement carries the volcanoes away from the hotspot. As a result, a trail of extinct volcanoes is left behind, forming a linear chain of islands. Each island in the chain represents a period of volcanic activity at that specific location as the plate moved over the hotspot.
Hotspot volcanism and the formation of island chains provide valuable insights into the dynamics of Earth's mantle and plate tectonics. By studying the age progression of islands in a chain, scientists can gain a better understanding of the movement and speed of tectonic plates and the behavior of mantle plumes deep beneath the Earth's surface.
Know more about Hotspot volcanism here:
https://brainly.com/question/12703688
#SPJ8
5. When 84 J of heat are added to 2.0 g of water at 15 C, what will be the final temperature?
Answers
When 84 J of heat are added to 2.0 g of water at 15°C the final temperature is 190.72°C
Heat is the transfer of kinetic energy from one medium or object to another from an energy source to a medium or object
Here given data is
heat are added = 84 J
Mass = 2.0 g
Initial temprature = 15°C
Then we have to calculate final temperature = ?
Formula for finding the final temprature is
Q = mc (T f -T i)
Q = heat = 84 J , m = mass = 2.0 g , T f = final temprature = ? , T i = initial temprature = 15°C , c = 4.184J/g°C
Q = mc (T f -T i)
T f = Q/mc + T i
T f = 84 J/2.0 g×4.184J/g°C + 15°C
T f = 190.72°C
Know more about temprature
https://brainly.com/question/16522925
#SPJ1
A student wants to study the effect of sunlight on plant growth. In his experiment, 12 plants
Answers
Answer:
the question isn't complete........................
The decomposition of ethanol (C2H5OH) on an alumina (Al2O3) surface was studied at 600 K. Concentration versus time data were collected for this reaction, and a plot of [A] versus time resulted in a straight line with a slope of -4.00 10-5 mol/L · s.
C2H5OH(g) → C2H4(g) + H2O(g)
(b) If the initial concentration of C2H5OH was 1.10 10-2 M, calculate the half-life for this reaction.
(c) How much time is required for all the 1.10 10-2 M C2H5OH to decompose?
Answers
The time that is taken is obtained as 5.21 × 10⁻⁵ s.
What is zero-order kinetics?For the zero order reaction that we have, we can see that;
rate = 24.00 × 10²⁵ M/s
From the integrated rate law:
[C₂H₅OH]t = [C₂H₅OH]₀ - 24.00 × 10²⁵ M/s × t
We now obtain the half life as;
t(1/2) = [C₂H₅OH]₀ / 2 × k
t(1/2) = (1.25 × 10²² M) / 2 × (24.00 × 10²⁵ M/s) = 2.60 × 10⁻⁵ s
The time that is required is now obtained as;
[C₂H₅OH]t = [C₂H₅OH]₀ - 24.00 × 10²⁵ M/s × t
0 M = 1.25 × 10²² M - 24.00 × 10²⁵ M/s × t
t = 5.21 × 10⁻⁵ s
The rate constant for the decomposition of ethanol on an alumina surface is 24.00 × 10²⁵ M.
The integrated rate law is: [C₂H₅OH]t = [C₂H₅OH]₀ - 24.00 × 10²⁵ M/s × t
If the initial concentration of C₂H₅OH was 1.25 × 10²² M, the half-life is 2.60 × 10⁻⁵ s.
The time required for all the 1.25 × 10²² M C₂H₅OH to decompose is 5.21 × 10⁻⁵ s.
Learn more about zero-order kinetics here: brainly.com/question/13314785
#SPJ1
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
2. What is the formula to calculate speed?
Answers
Answer:
s=d/t
s=speed
d=distance traveled
t=time elapsed
Which of these metals is the MOST reactive?
Lithium
Copper
Gold
Calcium
Answers
Answer:
The correct answer to you your question is Potassium
Explanation:
Potassium is the most reactive
2. What mass of NaOH is contained in 250.0 mL of a 0.400
M sodium hydroxide solution?
Answers
Answer:Explanation:
250.ml of a 0.400 divide
Calculate the amount of copper in moles in a 27.5g pure copper sheet
Answers
The amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To calculate the amount of copper in moles in a pure copper sheet, we need to use the molar mass of copper and the given mass of the sheet.
The molar mass of copper (Cu) is approximately 63.55 g/mol. This value represents the mass of one mole of copper atoms.
Given that the mass of the pure copper sheet is 27.5 g, we can calculate the number of moles using the following formula:
moles = mass / molar mass
Substituting the values:
moles = 27.5 g / 63.55 g/mol
moles ≈ 0.433 mol
Therefore, the amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To arrive at this result, we divided the given mass of the sheet (27.5 g) by the molar mass of copper (63.55 g/mol). This calculation allows us to convert the mass of the sheet into the corresponding number of moles of copper.
The result tells us that the 27.5 g pure copper sheet contains approximately 0.433 moles of copper atoms. This conversion to moles is useful in various chemical calculations and allows for easier comparison and analysis of quantities on a molecular scale.
for more such question on copper visit
https://brainly.com/question/29176517
#SPJ8
h-h+cl+cl____2h-cl... calculate the energy needed to break the bond
Answers
Write the name or the formula of the following compounds:
a. (Co(ClO3)4(NO2))Cl (N in NO2 is underlined)
b. K(Co(SO4)(NH2CH2CH2NH2)2(CN)) (N in CN is underlined)
c. sodium carbonyldiisothriocyanatotrinitritochromate(III)
b. amminediaquatrihydroxidochromium(VI) nitrate
Answers
The molecular formula for a molecular compound's molecular formula lists the variety of atoms that make up the molecule. The name of the compound (Co(ClO₃)₄(NO₂))Cl is tetrachloridobis(nitrito-O)cobalt(III) chloride.
The name or the formula of the following compounds are:
The name of the compound (Co(ClO₃)₄(NO₂))Cl is tetrachloridobis(nitrito-O)cobalt(III) chloride.
The name of the compound K(Co(SO₄)(NH₂CH₂CH₂NH₂)₂(CN)) is potassium bis(ethylenediamine)dihydroxidocyanidocobaltate(III) sulfate.
The formula of the compound sodium carbonyldiisothriocyanatotrinitritochromate(III) is Na[Cr(NCS)₂(NO₂)₃(CO)].
The formula of the compound amminediaquatrihydroxidochromium(VI) nitrate is [Cr(OH)₄(NH₃)₂(H₂O)₂]NO₃.
Learn more about formula, here:
https://brainly.com/question/30062954
#SPJ1
If others adopted similar habits, how might the world of science change?
Answers
Answer:
Explanation:
Smoking a cigarette, snorting cocaine, or drinking yourself into oblivion are all easy habits to adopt because they light up your brain with the neurotransmitter …
How many atoms are in 12 g of Carbon-12 (12C)?
Answers
There are approximately 6.022 × 10^23 atoms in 12 grams of Carbon-12 (12C).
The number of atoms in a given amount of a substance can be calculated using Avogadro's number, which represents the number of atoms or molecules in one mole of a substance. Avogadro's number is approximately 6.022 × 10^23.
Carbon-12 is a specific isotope of carbon, with an atomic mass of 12 atomic mass units (amu). One mole of Carbon-12 has a mass of 12 grams. Since one mole of any substance contains Avogadro's number of particles, in the case of Carbon-12, it contains 6.022 × 10^23 atoms.
Therefore, if we have 12 grams of Carbon-12, which is equal to one mole, we can conclude that there are approximately 6.022 × 10^23 atoms in this amount of Carbon-12.
In summary, 12 grams of Carbon-12 contains approximately 6.022 × 10^23 atoms. Avogadro's number allows us to relate the mass of a substance to the number of atoms or molecules it contains, providing a fundamental concept in chemistry and enabling us to quantify and understand the microscopic world of atoms and molecules.
for such more questions on atoms
https://brainly.com/question/6258301
#SPJ8
Which of the following statements about complete metamorphosis is TRUE?
The adult form of the animal is similar to the young animal.
Only arthropods undergo complete metamorphosis.
All organisms that undergo complete metamorphosis are a caterpillar at some time.
The physical form of the young animal is completely different than the physical form of the adult.
Answers
ill answer if u answer this FAST
pls and ty https://brainly.com/question/22200936
The true statement about metamorphosis is the physical form of the young animal is completely different from the physical form of the adult.
What is metamorphosis?Metamorphosis is the change in the structure of the animal in several stages until it changes into an adult form.
Animals that undergo metamorphosis are amphibians, insects, mollusk, crustaceans, fishes, etc.
The changes that occur in every stage are shape, nutrition form, behavior, etc.
The importance of metamorphosis is to change in every stage to reduce competition for food, predators, etc.
Thus, the correct option is D.
Learn more about metamorphosis
https://brainly.com/question/11651517
Determine the empirical formula of each of the following compounds from the percent composition: a. 7.8% carbon and 92.2% chlorine b. 10.0% C c. 0.80% H, 89.1% Cl
Answers
The empirical formula of a compound with 7.8% carbon and 92.2% chlorine is CCl₄ and that of a compound with 10.0% C, 0.80% H and 89.1% Cl is CHCl₃.
In order to determine the empirical formula of the compounds, we have to divide the percentage by mass of each of the elements by their relative atomic masses and then divide them through the lowest ratio.
Carbon = 7.8%
7.8/12 = 0.65
Chlorine = 92.2%
92.2 / 35.5 = 2.59
Dividing by the lowest ratio,
Carbon = 0.65/0.65 = 1
Chlorine = 2.59/0.65 = 3.98 ≅ 4
So the empirical formula is CCl₄.
Hydrogen = 0.80%
0.80/1 = 0.80
Chlorine = 89.1%
89.1/35.5 = 2.50
Carbon = 10.0%
10/12 = 0.83
Dividing by the lowest ratio,
Hydrogen = 0.80/0.80 = 1
Carbon = 0.83/0.80 = 1.03 ≅ 1
Chlorine = 2.50/0.80 = 3.1 ≅ 3
So the empirical formula is CHCl₃
To know more about empirical formula here
https://brainly.com/question/17409468
#SPJ4
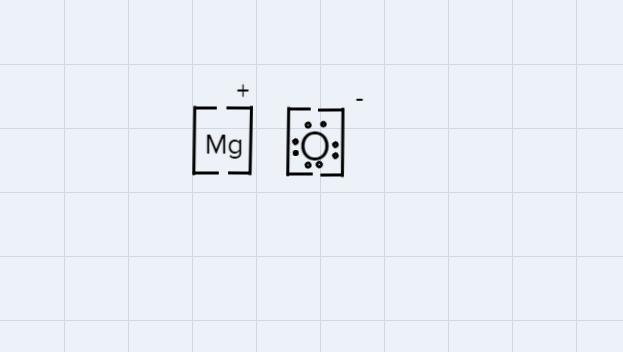
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?

Answers
Answer:
C.
Explanation:
The correct way to write the diagram of an ionic compound is:
- Use brackets by writing inside them, the symbol of each element separately.
- Draw the symbol and the number o the charge of each one, negative (-) add positive +()., in this case it is +1 for Mg (because Mg lost an electron) and -1 for O (because O won an elec).
Calculate the grams of C6H6 needed to produce 25 g of CO2
Show your work

Answers
Answer:
The given reaction is a combustion reaction of benzene,
C
6
H
6
. From its balanced chemical equation,
2
C
6
H
6
+
15
O
2
→
12
C
O
2
+
6
H
2
O
,
the mass of carbon dioxide
(
C
O
2
)
produced from 20 grams (g) of
C
6
H
6
is determined through the molar mass of the two compounds, given by,
M
M
C
O
2
=
44.01
g
/
m
o
l
M
M
C
6
H
6
=
78.11
g
/
m
o
l
and their mole ratio:
12
m
o
l
C
O
2
2
m
o
l
C
6
H
6
→
6
m
o
l
C
O
2
1
m
o
l
C
6
H
6
With this,
m
a
s
s
o
f
C
O
2
=
(
20
g
C
6
H
6
)
(
1
m
o
l
C
6
H
6
78.11
g
C
6
H
6
)
(
6
m
o
l
C
O
2
1
m
o
l
C
6
H
6
)
(
44.01
g
C
O
2
1
m
o
l
C
O
2
)
=
(
20
)
(
6
)
(
44.01
)
g
C
O
2
78.11
=
5281.2
g
C
O
2
78.11
m
a
s
s
o
f
C
O
2
=
67.6
g
C
O
2
Therefore, the mass in grams of
C
O
2
formed from 20 grams of
C
6
H
6
is
67.6
g
C
O
2
.
it is a problem of app
The grams of C₆H₆ needed to produce 25 g of CO₂ are 7.38g.
What is Mole concept?Avogadro's number is the number of units in one mole of any substance and equals to 6.02214076 × 10²³. The units can be electrons, atoms, ions, or molecules.
No. of moles is defined as a particular no. of particles that we can calculate with the help of Avogadro’s number.
Mass of a particular product is also find out by stoichiometry of a reaction as per the no. of mole given in the reaction.
Given,
Mass of CO₂ = 25g
2C₆H₆ + 15O₂ → 12CO₂ + 6H₂O
As per reaction, 156g of C₆H₆ gives - 528g of CO₂
0.295g of C₆H₆ gives - 1g of CO₂
0.295 × 25 g of C₆H₆ gives - 25g of CO₂ = 7.38g C₆H₆
Therefore, The grams of C₆H₆ needed to produce 25 g of CO₂ are 7.38g.
Learn more about Mole concept, here:
https://brainly.com/question/22540912
#SPJ7
Chlorine reacts with sodium and with hydrogen.
Compare the structure and bonding in sodium chloride and hydrogen chloride.
Answers
Answer:
Sodium Chloride has Ionic bond while Hydrogen Chloride has covalent bond.
Explanation:
Na has 11 electrons (2, 8, 1) and need to give away 1 electron to be stable
Cl has 17 electrons ( 2, 8, 7) and needs 1 electron to be stable.
Na transfers 1 electron to CL to form Ionic bond.
While
Hydrogen has 1 electron and shares with Chlorine to be stable.
Covalent bond involves sharing.


Chlorine reacts with sodium to form ionic sodium chloride while chlorine reacts with hydrogen to form covalent hydrogen chloride.
The nature of bonding between atoms depends on their relative difference in electronegativity.
Chemical bonding is the process by which atoms come together to form compounds.
There are different kinds of chemical bonds;
Ionic or electrovalent bondCovalent bondMetallic bondAn ionic bond is formed when there is high electronegativity difference between the bonding atoms. Typically, this type of bond is formed when the electronegativity difference is about 2.0 and above.
For a covalent bond, an electronegativity difference of about 0.4 - 1.7 is considered a polar covalent bond. Lower values of electronegativity difference corresponds to a nonpolar bond.
The electronegativity difference between sodium and chlorine is about 2.23. This corresponds to a pure ionic bond. The compound is composed of chlorine and sodium ion pairs alternately located in a crystal lattice as shown in the image attached.
The electronegativity difference between chlorine and hydrogen is about 0.96. This corresponds to a polar covalent bond. The negative end of the dipole points towards chlorine while the positive end of the dipole points towards hydrogen.
https://brainly.com/question/6071754

choose the hydration shells that form around a potassium ion when potassium chloride ( kcl ) dissolves. notice the positive, negative, and partial charges.
Answers
When KCl dissolves in water, the K⁺ ion forms a positive hydration shell surrounded by water molecules with partial negative charges, while the Cl⁻ ion forms a negative hydration shell surrounded by water molecules with partial positive charges.
When potassium chloride (KCl) dissolves in water, the potassium (K⁺) ion is surrounded by water molecules with partial negative charges on the oxygen atoms. This creates a hydration shell with a net positive charge, as the positive charge of the potassium ion is balanced by the partial negative charges of the surrounding water molecules.
On the other hand, the chloride (Cl⁻) ion is surrounded by water molecules with partial positive charges on the hydrogen atoms, creating an hydration shell with a net negative charge.
To learn more about hydration shells visit: https://brainly.com/question/10919810
#SPJ4
Describe a method that can be used to separate each of the following mixtures of metal ions.
a. Ag+, Pb2+
b. Mn2+, Fe3+
c. Ni2+, Al3+
d. Cu2+, Ni2+
Answers
A method that can be used to separate each of the following mixtures of metal ions. method use to separate the metal ion is the Qualitative analysis.
We have to use the Qualitative analysis for the separation of metal ion
a) Ag⁺ , Pb²⁺
group I cations produce insoluble chlorides and for precipitate with dilute HCl while all other cations remain soluble .
b) Mn²⁺ , Fe³⁺
group III cation produce very insoluble sulfides. by adding H₂S we can separate these ions. they can precipitate by high amount of sulfides ion.
c) Ni²⁺ , Al³⁺
these are also from group III , these can also be separated by adding acidic solution of H₂S.
d) Cu²⁺ , Ni²⁺
Group II cation is Cu²⁺ , they produced very insoluble sulfides . so they can be precipitate . so they cab be precipitate low amount of sulfides ion.
Thus, A method that can be used to separate each of the following mixtures of metal ions. method use to separate the metal ion is the Qualitative analysis.
To learn more about separation here
https://brainly.com/question/2516
#SPJ1
pls help will give brainilest or however you spell it

Answers
Answer:
1. balanced
2. balanced
3. unbalanced
4. balanced
5. balanced
6. unbalanced
7. balanced
8. unbalanced
Explanation:
hi!! can somebody help me with this question?
which is the best example of commensalism?
A. honey bees feed on the pollen of cornflowers and help the flowers reproduce
B. barnacles ride on a gray whales back to obtain food, and the whale is not harmed
C. squirrels and chipmunks eat the same nuts in a forest where food is scarce
D. prairie dogs often collect and eat food in groups so they are better protected from enemies
thx to anybody that helps! ♡︎
Answers
Answer:
B: Barnacles ride on a gray whale's back to obtain food, and the hale is not harmed.
Explanation:
I got it right on Edge.
i will give brainlelest to first to put a random answer just cuz i have like 600 points and i was bored so here is a nother one
Answers
Answer:
I mean okay
Explanation:
The height of three peaks; A, B, and C obtained in a gas chromatogram are 25.1 mm, 14.5 mm and 37.1 mm. The width of the peaks, at half height, are 4.6 mm, 4.4 mm and 5.9 mm, respectively. Calculate the percentage composition of A in the sample.
Answers
Answer: Percentage Compositon Of A in the sample= 28.99%
Explanation:
First we calculate the Peak area.
Because each peak is consdered like a triangle in a Chromatogram, The Area of the peak is calculated as = height x half height of the width
Area of Pea A= 25.1 mm X 4.6mm=115.46mm2
Area of Peak B= 14.5mm x 4.4mm=63.8mm2
Area of PeaK C= 37.1mm x5.9mm =218.89mm2
Total Peak Area= 398.15mm2
Percentage Area of Peak A = Area of Peak A/Total Peak Area x 100
= 115.46 / 398.15 x 100
=0.2899 x 100
=28.99%
Percentage Compositon Of A = 28.99%
What is the density of a piece of granite whose volume is 20 mL and mass is 53
grams?
3.05 g/mL
2.75 g/mL
4.0 g/mL
2.65 g/mL
Answers
2.65g/ml is the density of a piece of granite whose volume is 20 mL and mass is 53grams. Density is the mass of a specific material per unit volume.
What is density?Density is the mass of a specific material per unit volume. Density is defined as d = M/V, in which d represents density, M is weight, as well as V is volume. Density is generally expressed in grams every cubic centimetre. Water, for example, has a density of 1 gram per square centimeter, but Earth has a density of 5.51 kilograms per cubic centimetre.
Density is sometimes measured in kilos per cubic centimeter (in metre-kilogram-second or SI units). The density of air, for example, is 1.2 kilos per cubic metre.
density = mass / volume
=53/ 20
=2.65g/ml
Therefore, 2.65g/ml is the density of a piece of granite whose volume is 20 mL and mass is 53grams.
To learn more about density, here:
https://brainly.com/question/13434141
#SPJ1
What fraction of a 100 g sample of K - 42 will remain after 24.8 hours?
Answers
Answer:
1/4
Explanation:
From the question given above, the following data were:
Original amount (N₀) = 100 g
Time (t) = 24.8 h
Fraction remaining =?
NOTE: The half-life of K–42 is 12.4 h
Next, we shall determine the number of half-lives that has elapse. This can be obtained as follow:
Time (t) = 24.8 h
Half-life (t½) = 12.4 h
Number of half-lives (n) =?
n = t / t½
n = 24.8 / 12.4
n = 2
Finally, we shall determine the fraction remaining. This can be obtained as follow:
Number of half-lives (n) = 2
Fraction remaining =?
Fraction remaining = 1/2ⁿ
Fraction remaining = 1/2²
Fraction remaining = 1/4
15. The Uber vehicle travels for 6 hours at 75 mph. The Lyft vehicle travels for 8.5 hours at an average 62 n
Which driver covers more distance?
b) How far does the Uber driver drive?
c) How far does the Lyft driver drive?
Answers
Answer:
the uber driver
Explanation: