Which combination of elements would most likely form an ionic compound?
A sodium and fluorine
B. carbon and chlorine
C. hydrogen and oxygen
D. silicon and sulfur
Answers
Answer: not quite sure but I think A sounds best
Explanation:
Answer:
A. sodium and fluorine
Explanation:
Related Questions
an unknown hydrocarbon is subjected to elemental analysis. the results of this test show that the compound is 83.24% carbon and 16.76% h. with a molar mass of 72 g/mole, the molecular formula of this compound is:
Answers
The empiric molecular formula of this compound when an unknown hydrocarbon is subjected to elemental analysis is C4H9
C = 100.00 - 16.76 = 83.24% if H = 16.76%.
each percentage value by the corresponding atomic mass
H = 16.76/1 = 16.76
C = 83.24/12 = 6.94
Divide by a lesser number.
Remove fraction and multiply by 4 H = 9.64
C = 4 H = 16.76/6.94
= 2.41
C = 6.94/6.94 = 1
Empirical equation: C4H9
Octane, or (C4H9)2 (the hydrocarbon), could be the substance.
By calculating the number of moles of each element from its mass, empirical formulas are generated using empirically measured element masses. Obtaining subscripts for a possible empirical formula by dividing the molar amount of each element by its smallest molar amount.
For more information on empiric formula kindly visit to
https://brainly.com/question/14044066
#SPJ4
a student is using colored beads to make a model of aluminum sulfate, Al2(SO4)3.
Aluminum atoms are represented by green beads, sulfur atoms by blue beads, and
oxygen atoms by yellow beads. What combination of beads should the student use for
the model?
Answers
The combination of beads should the student use for the model :2 green, 3 blue and 12 yellow
the modelFurther explanationIn the chemical formula of a compound there are numbers:
1. The number indicates the number of atoms written after the element symbol
2. The number after the parentheses represents the number multiplied by the number of atoms in parentheses.
3. The number before the chemical formula shows the number of molecules behind it, which is usually used in equations
To calculate the beads needed to make a model, we must count the number of atoms of the Al₂(SO₄)₃ molecule
In the Al₂ (SO₄) ₃ molecule there are 3 atoms : Al, S, and O
1. Number of Al atoms: 2 2. The number of atoms S = 3 x 1 = 3 3. The number of atoms O = 4 x 3 = 12So the combination of beads
2 green(for Al), 3 blue(for S) and 12 yellow(for O)
Answer:
2 blue, 3 yellow, and 12 green
Explanation:
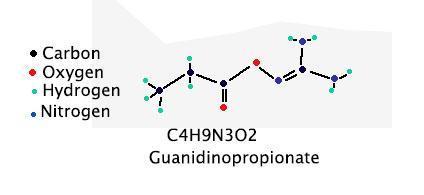
does someone know how to draw a structural formula of H1O2N3C4 please
Answers
Answer:
This could be guanidinopropionate. A drawing of the structure is attached.
Explanation:
There are other possible structures with this formula. The one shown in the attached diagram is one possibility. It is named, unfortunately, guanidinopropionate.
Which is true about the dissolving process in water?
A.Polar solutes do not dissolve easily in water.
B.Water molecules are attracted by solute ions at the surface of the solute.
C.Water molecules move throughout the solute.
D.Solute molecules pull water molecules away from the surface.
Answers
Answer:
this is what i know
Explanation:
Molecules or ions of a solute spread throughout the water molecules
b) why is the methyl group in toluene an ortho/para director? explain, using the reaction mechanism and drawings to support your answer. add an electrophile (you can use e ) to the ortho, meta, or para position,and compare the stabilities of the competing carbocation intermediates (the three possible sigma complexes).
Answers
The methyl group is toluene is not on meta position. The methyl group is electron releasing group.
In case of toluene, the methyl group connected to the ring, will increase the electron density at ortho and para positions via way of means of resonance with out converting some thing on the meta position. Thus the attacking reagent at once assaults on the electron wealthy site , accordingly the methyl institution is ortho directing. you may effortlessly apprehend the motion of electrons via way of means of following the route of arrows in structures. There isn't any shape wherein the electrons may be visible on meta position.
The resonating structures of toluene is attached below.
To learn more about resonance check the link below:
https://brainly.com/question/419650
#SPJ4

Waste products in the lab can always just be poured down the sink. True or false
Answers
Answer:
false
Explanation:
no, waste products in lab can't be poured down the sink because sometime cimicals can be harmfully and can damage the sink
Answer:
False
Explanation:
Your teacher has a designated area for getting rid of chemicals especially potentially hazardous chemicals
Omg please help me im doing so well in science i have a 100 and this if for a grade and i really need t o kkeep that 100

Answers
Answer:
b
Explanation:
This means that it takes more energy or heat to increase water's temperature than it does for most other substances. The specific heat of water is greater than that of dry soil, therefore water both absorbs and releases heat more slowly than land.
The pH of a solution is 0.50. To two decimal places, what is the hydrogen ion concentration of that solution
Answers
Explanation:
pH= –log¹⁰[H+]
0.50= –log¹⁰[H+]
log¹⁰[H+]=0.50
take the antilog of 0.50
[H+]= 3.16moldm-³
A set of solubility data is given below.
What is the mass of the dry solute
recovered?
Sample
2
Temperature
(°C)
30.1
Boat Mass
(8)
0.730
Boat +
Solution (g)
0.929
Boat + Dry
(g)
0.816

Answers
Answer:
0.086
Explanation:
got it on acellus
The mass of the dry solute recovered from the given data is 0.086 g. Option C
To determine the mass of the dry solute recovered, we need to subtract the mass of the boat from the mass of the boat with the dry solute.
Given the data provided:
Boat Mass: 0.730 g
Boat + Solution: 0.929 g
Boat + Dry: 0.816 g
To find the mass of the dry solute, we subtract the boat mass from the boat + dry mass:
Mass of Dry Solute = (Boat + Dry) - (Boat Mass)
Mass of Dry Solute = 0.816 g - 0.730 g
Mass of Dry Solute = 0.086 g
Therefore, the correct answer is c) 0.086 g.
The mass of the dry solute recovered from the given data is 0.086 g. It is important to note that the mass of the dry solute is obtained by subtracting the mass of the boat from the mass of the boat with the dry solute, as the boat mass represents the weight of the empty boat or container used in the experiment.
For more such questions on solute visit:
https://brainly.com/question/25326161
#SPJ8
What element has the noble gas electron configuration
[Kr]5524d105p?
Answers
Answer:
What is a noble gas electron configuration?
A noble gas electron configuration is a configuration that completes the Octet Rule of achieving 8 valence electrons.
Atoms always behave in ways to achieve stability and as you probably know, Noble Gases are the most stable. Their configuration, with a full valence electron shell (8 electrons, when you add both the S & P sublevels together, this is why it’s called the Octet), is therefore desirable. This means metals on the far left of the table will lose electrons to achieve this noble gas configuration and nonmetals on the right will gain electrons (generally speaking).
For example; take Argon. Its electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6, meaning it has 8 valence electrons. Then, take Chlorine. It has the electron configuration 1s^2 2s^2 2p^6 3s^2 3p^5, meaning it has 7 valence electrons so it’s a very unhappy camper. It typically gains an electron to achieve the 8 valence electrons Argon has (even using the same configuration of 1s^2 2s^2 2p^6 3s^2 3p^6) because it’s Mega jealous of Argon’ s stability. Side note: this is why chlorine typically has a -1 charge!
In summary, an atom achieving a “Noble Gas configuration” is the same as saying an atom fulfilling the Octet Rule. Both mean that there are 8 valence electrons (electrons in shell furthest from nucleus). This is a stable form many atoms seek to achieve (of course, what’s a good rule in chemistry if there aren’t exceptions!).
Which is the strongest base?
A. NaOH
B. Mg(OH)2
C. NH4OH
D. None of these
Answers
In terms of strength, NaOH (sodium hydroxide) is considered the strongest base. So, the correct answer is Option A.
This is because it is a strong electrolyte and dissociates completely in water to produce hydroxide ions (OH-). This results in a high concentration of hydroxide ions and a high pH value, making it a strong alkaline substance.
Mg(OH)2 (magnesium hydroxide) is also considered a strong base, but not as strong as NaOH. It dissociates partially in water, producing hydroxide ions, but not as many as NaOH.
NH4OH (ammonium hydroxide) is a weak base, as it only partially dissociates in water, producing hydroxide ions. The concentration of hydroxide ions is not as high as NaOH, making it less alkaline and weaker in terms of strength.
So, the correct answer is Option A.
To read more about strongest base, Visit-
https://brainly.com/question/24003853
#SPJ11
Calculate the pH during the titration of 20.00 mL of 0.1000 M ammonia, with 0.2000 M HCl(aq) after 6.07 mL of the acid have been added.
Answers
When an acid is added to an alkaline solution such as ammonia, the pH of the solution decreases. This is because hydrogen ions, which are acidic, are added to the solution. The pH of the solution is calculated using the formula pH = -log[H+].Given information:Initial volume of ammonia solution = 20.00 mLInitial concentration of ammonia solution = 0.1000 MVolume of HCl added = 6.07 mL Concentration of HCl solution = 0.2000 M1.
Write the balanced chemical equation for the reaction that takes place during the titration of ammonia with hydrochloric acid. NH3 + HCl → NH4Cl2. Calculate the number of moles of ammonia present initially. n = C x V where ,n = number of mole C = concentrationV = volume Substituting the values, we getn(NH3) = 0.1 mol/L x (20.00/1000) L= 0.002 mol3. Calculate the number of moles of HCl added. n(HCl) = C x Vwhere,n = number of moles C = concentrationV = volumeSubstituting the values, we getn(HCl) = 0.2 mol/L x (6.07/1000) L= 0.001214 mol4. Calculate the number of moles of ammonia remaining. At this stage, all the hydrochloric acid added will react with ammonia to form ammonium chloride. The amount of hydrochloric acid added is less than the amount required to completely react with ammonia. Hence, some ammonia will remain unreacted.n(NH3) remaining = n(initial) - n(HCl added)= 0.002 - 0.001214= 0.000786 mol5. Calculate the volume of hydrochloric acid added when all the ammonia has reacted. From the balanced equation, the stoichiometry of ammonia and hydrochloric acid is 1:1. Hence, the number of moles of ammonia that has reacted is equal to the number of moles of hydrochloric acid added. n(HCl) reacted = 0.000786 moln(HCl) added at equivalence point = n(HCl) reacted + n(HCl) initially= 0.000786 + 0.001214= 0.002 molV(HCl) added at equivalence point = n(HCl) / CV(HCl) = 0.002 mol / 0.2 mol/L= 0.01 L or 10 mL6. Calculate the pH after 6.07 mL of hydrochloric acid has been added. At this stage, the number of moles of hydrochloric acid added is less than the number of moles required to reach equivalence point. Hence, the hydrochloric acid added is limiting, and the pH is calculated using the Henderson-Hasselbalch equation.pH = pKa + log([base]/[acid])At this stage, ammonia is the base and ammonium chloride is the acid.pKa for NH4+/NH3 = 9.25[base] = n(NH3) remaining = 0.000786 mol[acid] = n(HCl added) = 0.001214 molSubstituting the values, we getpH = 9.25 + log(0.000786/0.001214)= 9.25 - 0.121= 9.13Therefore, the pH of the solution after 6.07 mL of hydrochloric acid has been added is 9.13.For such more question on mole
https://brainly.com/question/29367909
#SPJ11
If a student measured an object's density to be 2.70 g/mL, 2.32 g/mL, 2.12 g/mL and 3.88, then his results would considered ______The object's actual density is 3.50 g/mL A. Accurate and precise B.Precise but not accurate C. Neither accurate nor precise D.Accurate but precise
Answers
Answer:
C. Neither accurate nor precise
Explanation:
The student's results would be considered precise if they were close to one another. However they vary significantly from one another.Regarding accuracy, they would be considered accurate if they were close to the actual value. The given results are too far away from the actual value.Thus the results are neither accurate nor precise.
list the four different sublevels and (given that only a maximum of two electrons can occupy an orbital) determine the maximum number of electrons that can exist in each sublevel.
Answers
l = 0 → s = 2 electrons, l = 1 → p = 6 electrons, l = 2 → d = 10 electrons, l= 3 → f = 14 electrons.
What is electron?The smallest elemental component of an atom, the electron has a negative charge. The smallest elemental component of an atom, the electron has a negative charge. In a negative ion, there are an adequate amounts of both electrons and protons.
Briefing:According to the subatomic particles, the orbit region of space has a higher likelihood of harboring an electron. It is impossible to simultaneously detect the electron's location and velocity (uncertainty principle). Therefore, the theory establishes five electron density to represent one electron, making it simple to recognize it:
→n is the principal quantum number and identify the shell where the electron is.It ranges from 1 to 7, and the consonants K, L, m actually, N, O, P, etc Q stand in for it;
→l is the azimuthal quantum number and identify the subshell (or sublevel) where the electron is. The consonants s, p, d, or f stand in for it, which ranges from 0 to 3;
→ml is the magnetic quantum number, and it represents the orbital. It varies from -l to +l, passing by 0. There can be at least two electrons in each orbital;
→ms is the spin number and represents the spin of the electrons. The range is +1/2 to -1/2.
The sublevel s (l = 0) only has one orbital, enabling it to have at least two electrons; the subarea p (l = 1) has three orbitals, so it can and has at least six electrons; the subarea d (l = 2) has five orbitals, so it can would have had at least ten electrons; and the basement level f (l = 3) has seven orbitals, enabling it to have at least fourteen electrons.
To know more about Electrons visit:
https://brainly.com/question/18367541
#SPJ4
Why Atomic size decreases as we go from left to right in Modern Periodic Table?
Answers
Answer:
There is an increase in nuclear charge.
in your own words describe protons, neutrons, and electrons *
Answers
is the lightest essential particle that makes up an atom and has the least possible charge in terms of negative electricity.
A protonis a subatomic particle with a positive electrical charge that, together with neutrons, forms the nucleus of atoms.
A neutronis a massive particle with no electrical charge. It is a baryon made up of two down quarks and one up quark.
Plzzzz Help!!!!
What is the molarity of a solution that contains 1.45 moles of KCl in 1, 250 mL of
solution?
Answers
Molarity of the solution is 5.8M
Given :
moles of solute (n) = 1.45
Volume of solution (V) = 250 ml
WHAT IS MOLARITY ??
Molarity of a solution is defined as the number of moles of the solute dissolved per litre of the solution. It is represented as M . Thus a solution, which contains one gram mole of the the solution dissolved per litre of the solution is regarded as .
M = n of solute / Vol (L)
= \(\frac{1.45 * 1000}{100 * 250}\) = 5.8 M
Learn more about molarity at https://brainly.in/question/1159016
#SPJ2

What is the work done when a force of 5 N is applied to a ball and it moves 8000 cm?
Answers
Answer:
Explanation:Given;
applied force, F = 8000 N
time of force application, t = 15 s
Work done is given as the product of force and displacement. Since the car is unable to move, then the displacement is zero and the work done is zero.
Work done = Force x displacement
Work done = 8000 N x 0
Work done = 0
Therefore, the work done is zero.
consider the reaction below. h2po4– h2o h3o hpo42– which of the following is a base–conjugate acid pair? h2o and h3o h2o and h2po4– h2po4– and hpo42– h2po4– and h3o
Answers
The reaction given below can be represented as follows:`
H2PO4– + H2O ⇋ H3O+ + HPO42–
`Base–conjugate acid pair:` H2PO4–` and `HPO42–`
A Brønsted-Lowry acid-base reaction involves the transfer of a proton from one substance to another.
In this reaction, H2PO4– acts as an acid, donating a proton to water.
This produces the conjugate base of H2PO4–, HPO42–, and the conjugate acid of water, H3O+.
A base-conjugate acid pair is defined as two substances that differ only in the presence or absence of a single proton.
In this reaction, the base H2PO4– loses a proton to form its conjugate acid, HPO42–.
Therefore, `H2PO4–` and `HPO42–` is the base–conjugate acid pair.
The term "base-conjugate acid pair" refers to two species with the following characteristics:
They are both conjugates.
They differ by one hydrogen ion.
The dissociation of an acid (HA) results in the formation of a conjugate base (A−) and a hydrogen ion (H+). HA is called an acid, while A− is called a conjugate base.
Learn more about conjugate acid at: https://brainly.com/question/12584785
#SPJ11
The fact that water is attracted to itself, a property called:.
Answers
what is the hydrogen ion concentration in a blood sample that registers a ph of 7.30 using a ph meter?
Answers
The hydrogen ion concentration in a blood sample with a pH of 7.30, as measured by a pH meter, is approximately \(5.01 x 10^(-8) M\). This value indicates a slightly acidic blood sample, which may be outside the typical range for healthy individuals.
The pH is a measure of the hydrogen ion concentration (H+) in a solution. The pH scale ranges from 0 to 14, with a pH of 7 being neutral. The formula to calculate hydrogen ion concentration from pH is:
\(H+ = 10^(-pH)\)
In the context of a blood sample, a pH meter is used to measure the pH of the blood. The pH of healthy human blood typically falls within the range of 7.35 to 7.45, with a pH of 7.30 indicating slightly acidic blood.
Using the given pH value of 7.30, we can calculate the hydrogen ion concentration as follows: \(H+ = 10^(-7.30)\), \(H+ ≈ 5.01 x 10^(-8) M (molar)\)
This means that the blood sample has a hydrogen ion concentration of 4.47 x 10^-8 mol/L. It's worth noting that even small changes in pH can have significant effects on biological systems, including enzyme activity and protein structure. The normal pH range of human blood is tightly regulated between 7.35 and 7.45,
Know more about pH scale here:
https://brainly.com/question/1433865
#SPJ11
A chemical reaction causes the chemical compositions of substances to change. Reactants are substances that enter into a reaction, and products are substances produced by the reaction
Which of the following are the reactants for this simulation? Select all that apply.
Answers
The statements which is true about the reactants from the given simulation above is: It involves the breaking of chemical bonds.
The correct answer choice is option c
How does reactants involved in the breaking of chemical bonds?For a chemical reaction to occur, the bond between the reacting substances breaks either by covalent or electrovalent bonding in order to form new substance.
So therefore, reactants usually break chemical bonds duringchemical reaction.
Complete question:
A chemical reaction causes the chemical compositions of substances to change. Reactants are substances that enter into a reaction, and products are substances produced by the reaction
Which of the following are the reactants for this simulation? Select all that apply
a. A chemical reaction results to formation of new bonds
b. A chemical reaction involves the formation of new substance.
c. It involves the breaking of chemical bonds
Read more chemical reactions: https://brainly.com/question/5024599
#SPJ1
how would separate a mixture of lead 2 chloride and sodium chloride
Answers
Answer:
lower th
Explanation:
Pb{Cl}_{2})$ is an inorganic
answer:
lower the temperature so the lead chloride settles out of solution
compound which is a white solid under ambient conditions. It is one of the most important lead-based
reagents. It is poorly soluble in water. And it is insoluble in cold water. Sodium chloride is ssolble at all temperature
so lower the temperature
What is the relationship between absorbance and energy?
Answers
The relationship between absorbance and energy is the minimum energy for photoactivation (Ea) is to the wavelengths of the peak absorbance.
The Absorbance will be related to the amount of the substance in the solution, therefore it can be used to the quantitatively determination of the amount of the substance that will be present. The shorter the wavelength, the greater the energy.
The Absorbance can be defined as the logarithm of the ratio of the incident to the transmitted radiant power through the sample. Energy of the absorption can be defined, the surface below the load displacement curve. The minimum energy for photoactivation to wavelengths of the peak absorbance.
To learn more about absorbance here
https://brainly.com/question/29750964
#SPJ4
How does an emerging idea differ from scientific consensus? Which best describes emerging scientific ideas?
Answers
Emerging scientific ideas are new theories or ideas that are gaining attention in the scientific community, but have not yet been fully accepted or confirmed.
Emerging ideas refer to the new and innovative ideas or theories that have yet to gain full scientific acceptance. While a scientific consensus is a view or theory that has been universally accepted and confirmed by multiple experiments or research, an emerging scientific idea is a new and unproven theory or idea that is gaining attention in the scientific community. These emerging ideas may also be referred to as scientific hypotheses. In contrast to scientific consensus, emerging scientific ideas have not yet been subjected to rigorous testing and confirmation.
They are generally proposed to explain new observations or experimental results, which have not yet been fully understood or explained by established scientific theories. Emerging scientific ideas can have the potential to challenge the current scientific consensus. If an emerging scientific idea is found to be valid, it can ultimately lead to the establishment of a new scientific consensus. For example, the emerging scientific idea of the Higgs boson particle led to the discovery of a new field in particle physics, which is now an established scientific consensus.
for such more questions on scientific
https://brainly.com/question/29886197
#SPJ8
Why are fat layers and thick white fur advantageous for polar bears?
Group of answer choices
A. They insulate the bears and allow them to blend in with their environment.
B. They help the bears get rid of excess body heat more readily.
C. They help bears hibernate longer.
D. They allow the bears to run and swim faster to escape predators.
Answers
Answer:
A. They insulate the bears and allow them to blend in with their environment
Water and salt are compounds made of the combination of more than one type of atom. Which statement is false?
Answers
Answer:
This statement is correct
Explanation:
Water is made up of 2 hydrogen atoms and 1 oxygen atom while salt is made up of 1 atom of sodium and 1 atom of chloride
:)
Calculate the molarity of a solution containing 0.2 mol of sodium hydroxide dissolved in 0.5 L of water. Be sure to report your answer in proper significant figures and use the appropriate symbol.
Answers
Answer:
The molarity is 0.4 \(\frac{moles}{liter}\)
Explanation:
The Molarity (M) or Molar Concentration is a concentration unit that indicates the number of moles of the solute per liter of solution. In other words, molarity is defined as the number of moles of solute that are dissolved in a given volume.
The Molarity of a solution is determined by the expression:
\(Molarity (M)=\frac{number of moles of solute}{volume}\)
Molarity is expressed in units (\(\frac{moles}{liter}\)).
In this case:
number of moles of solute (sodium hydroxide)= 0.2 molesvolume= 0.5 LReplacing:
\(Molarity (M)=\frac{0.2 moles}{0.5 L}\)
Molarity= 0.4 \(\frac{moles}{liter}\)
The molarity is 0.4 \(\frac{moles}{liter}\)
TRUE/FALSE. To insure proper operation of an oxygen cylinder regulator, make sure no oil residue is present.
Answers
"To insure proper operation of an oxygen cylinder regulator, make sure no oil residue is present" is true because oil residue can compromise the functioning of an oxygen cylinder regulator.
Explanation: It is true that to ensure proper operation of an oxygen cylinder regulator, it is important to make sure that no oil residue is present. Oxygen cylinders contain highly concentrated oxygen that can react violently with oil or grease, leading to combustion or explosions. Oil residue can contaminate the regulator and pose a significant safety risk.
Therefore, it is crucial to keep oxygen cylinders and their regulators free from any oil or grease to maintain safe and proper functioning.
To learn more about oxygen cylinders, Visit:
https://brainly.com/question/29016884
#SPJ11
an athlete has 15% body fat by mass. What is the weight of fat, in pounds, of a 74 kg-athlete?
Answers
The weight of fat is 24.27 pounds of a 74 kg athletes.
How to find the weight of substance ?15% by mass means 15 g of fat is present in 100g of mass.
Convert kg into grams
1 kg = 1000 gram
74 kg = 74000 g
Now,
Mass of body = \(\frac{74000 g \times 15\ g}{100}\)
= 740 × 15
= 11100 g
Convert g into kg
11100 g = 11.1 g
Convert kg into pounds
1 kg = 2.205 pounds
11.1 kg = 11.1 × 2.205
= 24.47 pounds
Thus from above conclusion we can say that the weight of fat is 24.27 pounds of a 74 kg athletes.
Lean more about the Weight of substance here: https://brainly.com/question/7152617
#SPJ2