Pls help! Urgent!
The law of conservation of mass states that the mass of the reactants is _____
the mass of the products.
A. Less than
B. Equal to
C. Greater than
Answers
Answer:
c
Explanation:
Answer:
b. equal too
Explanation:
According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants
Related Questions
In a presentation about measuring mass, one of your classmates states, "Two objects of the same size will always have the same mass.” Is this statement correct? Why or why not?
Answers
Answer:
The statement is not correct.
Explanation:
If two objects have the same mass, their respective densities determine their volumes. By definition, density = mass/volume or volume = mass/density. Therefore if two objects have the same mass, the mode dense object (with higher density) will occupy less volume than the other object.
C. Use the data provided in Table 2 to complete the following.
Sketch a phase diagram for O₂. The diagram should be roughly to
scale and include the Triple point and Critical point.

Answers
The triple point can be seen from the graph that has been attached here.
What is the triple point?The triple point is a special combination of temperature and pressure where the equilibrium of a substance's three phases—solid, liquid, and gas—occurs. The transition between phases happens with no discernible net change in the substance because the solid, liquid, and gas phases are in perfect equilibrium at the triple point.
A crucial reference point in thermodynamics, the triple point is frequently used to specify temperature scales and calibrate thermometers. Each substance has a specified value for temperature and pressure.
Learn more about triple point:https://brainly.com/question/29017350
#SPJ1

Name three silicon wafer cleaning methods and compare their
efficacy
Answers
RCA cleaning, SC1/SC2 cleaning, and megasonic cleaning are the three silicon wafer cleaning methods. Each of them have their advantages and are commonly used in semiconductor manufacturing processes.
There are several methods used to clean silicon wafers in the semiconductor industry.
Here are three common methods along with a comparison of their efficacy:
1) RCA Cleaning (Radio Corporation of America):
RCA cleaning is a widely used method for silicon wafer cleaning. It involves a two-step process:
a. RCA-1: The wafer is immersed in a mixture of deionized water, hydrogen peroxide (H₂O₂), and ammonium hydroxide (NH4OH). This step removes organic contaminants, particles, and some metal ions from the wafer surface.
b. RCA-2: The wafer is then immersed in a mixture of deionized water, hydrogen peroxide, and hydrochloric acid (HCl). This step removes metallic and ionic impurities from the wafer surface.
Efficacy: RCA cleaning is highly effective in removing organic and inorganic contaminants. It provides a good level of cleanliness for most semiconductor fabrication processes.
2) SC1 and SC2 Cleaning (Standard Clean 1 and Standard Clean 2):
SC1 and SC2 cleaning are alternative methods to RCA cleaning and are used for wafer surface preparation. The process involves the following steps:
a. SC1: The wafer is immersed in a mixture of deionized water, hydrogen peroxide, and ammonium hydroxide. This step removes organic and ionic contaminants from the wafer surface.
b. SC2: The wafer is immersed in a mixture of deionized water, hydrogen peroxide, and hydrochloric acid. This step removes metallic and oxide contaminants from the wafer surface.
Efficacy: SC1 and SC2 cleaning methods are effective in removing various types of contaminants from the wafer surface. They provide comparable cleanliness to RCA cleaning.
3) Megasonic Cleaning:
Megasonic cleaning involves the use of high-frequency sound waves (usually in the range of 800 kHz to 2 MHz) to agitate the cleaning solution and remove particles from the wafer surface. It is often used in conjunction with RCA or SC cleaning methods.
Efficacy: Megasonic cleaning is highly effective in removing particles from the wafer surface. It can dislodge and remove smaller particles that may be difficult to remove by chemical cleaning methods alone.
Learn more about Ammonium Hydroxide at
brainly.com/question/14991293
#SPJ4
An unknown compound has the following chemical formula: PbO where x stands for a whole number. Measurements also show that a certain sample of the unknown compound contains 3.1 mol of oxygen and 3.11 mol of lead. Write the complete chemical formula for the unknown compound. ៣ 00 X S
Answers
An unknown compound has the following chemical formula: PbO where x stands for a whole number. Measurements also show that a certain sample of the unknown compound contains 3.1 mol of oxygen and 3.11 mol of lead. The complete chemical formula for the unknown compound is PbO, indicating that for every mole of lead, there is one mole of oxygen
To determine the complete chemical formula for the unknown compound with the formula PbO, we need to find the whole number value of x by using the given measurements.
According to the chemical formula, PbO, the ratio of lead (Pb) to oxygen (O) is 1:1. This means that for every mole of lead, there is one mole of oxygen.
Given that the sample contains 3.1 mol of oxygen and 3.11 mol of lead, we can compare the moles of oxygen and lead to determine the whole number value of x.
From the given measurements, we observe that the ratio of oxygen to lead is approximately 3.1:3.11. To simplify this ratio, we can divide both values by the smaller one, which is 3.1.
3.11 mol of lead / 3.1 mol of oxygen = 1 mol of lead / 1 mol of oxygen
This indicates that the whole number value of x in the chemical formula PbO is 1.
Therefore, the complete chemical formula for the unknown compound is PbO, indicating that for every mole of lead, there is one mole of oxygen.
Learn more about mole here:
https://brainly.com/question/30885025
#SPJ11
A plastic sphere floats in water with 50. 0% of its volume submerged. This same sphere floats in glycerin with 40. 0% of its volume submerged. Determine the density of the sphere.
Answers
The density of the sphere is 1/2 of water density
The mass of a substance per unit of volume is the definition of density. Although the sign can also represent the Latin letter D, it is most frequently used to represent density. The density of a substance is a measurement of how firmly it is packed. Given that it is the mass per unit volume, it has this definition. D or Density is a density indicator. Formula: = m/V is the formula, where m is the object's mass and V is its volume.
Density is the quantity of mass per unit of volume and is defined as the ratio of mass to volume.
A plastic spherical floats in the water when 50% of its volume is submerged because of this. Thus, sphere density equals half of water density.
Learn more about density at brainly.com/question/29775886
#SPJ4
The primary function of a chloroplast is to -
F . Convert food into energy a cell can use
G . Control the function of a cell and control genetic traits
H . Convert radiant energy into chemical energy through photosynthesis
J . Store water and nutrients
Please help!!!
Answers
H. Convert radiant energy into chemical energy through photosynthesis
stoichiometry question… how do i do these 2 problems??

Answers
The mass of magnesium chloride produced is 179.15 grams.
The balanced chemical equation for the reaction is:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
From the equation, we can see that 1 mol of Mg reacts with 2 mol of HCl to produce 1 mol of MgCl₂ and 1 mol of H₂.
So, to determine the limiting reactant, we need to calculate the moles of MgCl₂ that can be formed from each reactant:
Moles of MgCl₂ from Mg = 3.79 mol Mg × (1 mol MgCl₂/1 mol Mg) = 3.79 mol MgCl₂
Moles of MgCl₂ from HCl = 3.75 mol HCl × (1 mol MgCl₂/2 mol HCl) = 1.88 mol MgCl₂
Since the amount of MgCl₂ that can be formed is limited by the amount of HCl available, HCl is the limiting reactant.
The stoichiometry of the reaction tells us that 2 moles of HCl react to produce 1 mole of MgCl₂. Therefore, the number of moles of MgCl₂ produced in the reaction is:
1.88 mol MgCl2
Finally, we can use the molar mass of MgCl2 to calculate the mass produced:
Molar mass of MgCl₂ = 95.211 g/mol
Mass of MgCl₂ produced = 1.88 mol × 95.211 g/mol = 179.15 g.
Learn more about magnesium chloride here:
https://brainly.com/question/30671024
#SPJ1
A reaction requires 22.4 L of gas at STP. You have 50.0 L of gas at 101.5 kPa and 373 K.
Which statement is true?

Answers
Answer: D: You will have excess gas for this reaction
Explanation: I just took the test
1. What type of reaction is this?
H2O + H2 + O2
A. Synthesis
B. Decomposition
C. Combustion
D. Single Replacement
E. Double Replacement
I say synthesis
Answers
Answer:
B if you meant \(H_{2}O\)->\(H_{2} +O_{2}\)
Explanation:
A. Synthesis: reaction where two or more reactants combine to form one product. A+B->AB
B. Decomposition: reaction where a single compound reacts to form more than one product. AB-> A+B
C. Combustion: any reaction in which a substance reacts with oxygen gas. (needs \(O_{2}\))
D. Single replacement: a type of reaction where one element replaces a similar element within a compound. Reaction is always an element and a compound.
E. Double replacement: a type of reaction where the ions of two compounds exchange places in an aqueous solution to form two new compounds. AB+CD->AD+CD
5.00 mL of base is added to the 15.00 mL of acid in previous problem what is the ph of the resulting solution containing 0.0002 moles of hydrogen ions
Answers
The pH of the resulting solution containing 0.0002 moles of hydrogen ions and a total volume of 20.00 mL is approximately 2.30.
To calculate the pH of the resulting solution, we first need to determine the concentration of hydrogen ions (H+) in the solution.
Since there are 0.0002 moles of H+ ions in a total volume of 20.00 mL (15.00 mL acid + 5.00 mL base), we can find the concentration by dividing the moles of H+ ions by the total volume in liters (0.02 L).
The concentration of H+ ions is 0.0002 moles / 0.02 L = 0.01 M.
Next, we can use the pH formula: pH = -log[H+].
By plugging in the concentration of H+ ions, we get pH = -log(0.01) which results in a pH of approximately 2.30.
Learn more about hydrogen ions here:
https://brainly.com/question/20309096
#SPJ11
Using the rules for significant figures, what do you get when you multiply 67.6 by 1.2?
Answers
Answer:
The answer should have as many significant figures as the lowest number used in the multiplication, which is 1.2 .
BRAINLIEST!! HELP!! The pure silicon used in silicon computer chips can be generated by the reaction of silicon tetrachloride with magnesium metal which generates solid silicon and solid magnesium chloride. You react 1.0 moles of silicon tetrachloride with 5.0 moles of magnesium metal and your target product is silicon.
Equation: ?
Limiting reactant; ?
Moles of target product produced: ?
Moles of excess reagent remaining: ?
Answers
Reaction
SiCl₄+ 2Mg ⇒ Si + 2MgCl₂
to find limiting reagents: divide the number of moles by their coefficients
SiCl₄ = 1 mole : 1 (coefficient) = 1
Mg = 5 mole : 2 (coefficient) = 2.5
SiCl₄ as a limiting reactant (smaller), and Mg as an excess
then all the compounds involved in the reaction are based on moles of SiCl₄
Si produced =
\(\tt \dfrac{coefficient~Si}{coefficient~SiCl_4}\times mole~Si=\dfrac{1}{1}\times 1=1\)
Reacted Mg (excess) = 2/1 x 1 = 2 mole
mole Mg remaining = 5 - 2 = 3 mole
A 35. 3g of element M is reacted with nitrogen to produce 43. 5g compound M3N2, what is the molar mass of the element
Answers
The molar mass of element M can be calculated based on the given mass of the compound \(M_{3}N_{2}\) formed from the reaction. In this case, the molar mass of element M is 12.01 g/mol.
To calculate the molar mass of element M, we need to use the law of conservation of mass. The mass of the compound M_{3}N_{2} (43.5 g) is equal to the sum of the masses of three atoms of M and two atoms of nitrogen. We can calculate the mass of M in the compound by subtracting the mass of nitrogen from the total mass.
The molar mass of nitrogen (N) is 14.01 g/mol. Therefore, the total mass of nitrogen in the compound is 14.01 g/mol × 2 = 28.02 g/mol.
Now, subtracting the mass of nitrogen from the total mass of the compound, we get the mass of M: 43.5 g - 28.02 g = 15.48 g.
Since the mass of 35.3 g of element M reacted to form 15.48 g of M in the compound, we can conclude that the molar mass of element M is 15.48 g/mol / 35.3 g/mol = 12.01 g/mol, which is the molar mass of carbon (C).
Learn more about compound here: https://brainly.com/question/27513728
#SPJ11
Assuming that the trends continue, which of the following compound will have the greatest solubility at 120 ℃? The graph below shows the solubility of a variety of compounds.
A. Ce2(SO4)3
B. K2Cr2O7
C. NaCl
D. Pb(NO3)2
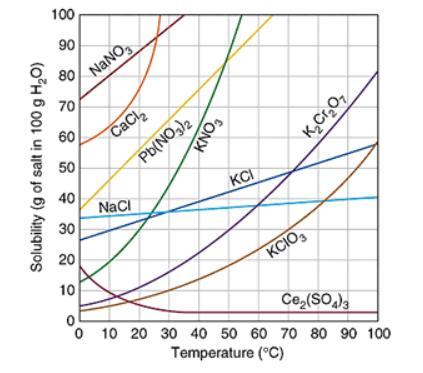
Answers
Answer:
its c
Explanation:
i took the test and got a 100
Assuming that the trends continue, sodium chloride compound will have the greatest solubility at 120 ℃.
What is solubility?Solubility is defined as the ability of a substance which is basically solute to form a solution with another substance. There is an extent to which a substance is soluble in a particular solvent. This is generally measured as the concentration of a solute present in a saturated solution.
The solubility mainly depends on the composition of solute and solvent ,its pH and presence of other dissolved substance. It is also dependent on temperature and pressure which is maintained.Concept of solubility is not valid for chemical reactions which are irreversible. The dependency of solubility on various factors is due to interactions between the particles, molecule or ions.
Learn more about solubility,here:
https://brainly.com/question/22185953
#SPJ3
Marco was looking at this picture of two boats sitting differently in the water. He decided to compare the way the two boats sit in the water to the way land is behaving in Greenland.
Answers
I don't know
Explanation:
because it does not give an question
If you want to prepare 2.00 L of a 0.800 M NaNO_3 solution from a NaNO_3 stock solution that is 1.5 M in concentration, how many mL of the stock solution must you start with
Answers
1066.7 ml of the stock solution must start with, preparing 2.00 L of a 0.800 M NaNO3 solution.
What is volume?Volume is the space occupied by a three-dimensional object.
By the formula \(\rm M_1V_1= M_2V_2\)
M1 is the initial molarity, 1.5 M
M2 is the final molarity, 0.800 M
V1 is the initial volume
V2 is the final volume, 2.00 L
Putting the values in the equation
\(\rm 1.5\;M \times V_1= 0.800\;M \times 2.00\;L\\\\V_1 = \dfrac{ 0.800\;M \times 2.00\;L}{1.5\;M} = 1066.7\;ml\)
Thus, the initial volume is 1066.7 ml
Learn more about volume
https://brainly.com/question/1578538
#SPJ1
How well does the number of beers a student drinks predict his or her blood alcohol content? Sixteen student volunteers at Ohio State University drank a randomly assigned number of cans of beer. Thirty minutes later, a police officer measured their blood alcohol content (BAC). The data are in the file p:\data\math\hartlaub\Elements of Statistics\bac.csv. The students were equally divided between men and women and differed in weight and usual drinking habits. Because of this variation, many students don't believe that the number of drinks predicts blood alcohol well.Make a scatterplot of the data. Find the equation of the least-squares regression line for predicting blood alcohol from number of beers and add this line to your plot. What is r-squared for these data? Briefly summarize what your data analysis shows.
Answers
Scatterplot: To visualize the relationship between these two variables, create a scatterplot of the number of beers versus blood alcohol content (BAC).
The next step would be to find the equation of the least-squares regression line, which would give the best prediction of BAC based on the number of beers. A regression analysis software or a statistical formula can be used to calculate the equation.
R-squared is the coefficient of determination, and it measures the strength of the linear relationship between two variables. A strong linear relationship is indicated by a high R-squared value, whereas a weak relationship is indicated by a low R-squared value.
The data analysis demonstrates the relationship between the number of beers and the BAC, as well as the accuracy of the prediction made using the least-squares regression line. The R-squared value provides information on the strength of this relationship, which helps determine the usefulness of using the number of beers to predict BAC.
It's important to note that this analysis should be interpreted with caution, as factors such as body weight, gender, and other individual characteristics can also affect BAC levels.
To Learn More About data analysis click
https://brainly.com/question/13103333
#SPJ4
Any compound that increases the number of hydronium ions when dissolved in water is called?
Answers
A compound that increases the number of hydronium ions when dissolved in water is known as an acid.
Acids are compounds that donate protons (H+) to water molecules, resulting in the formation of hydronium ions (\(H_3O^+\)). Acids have a sour taste, react with metals to produce hydrogen gas, turn blue litmus paper red, and have a pH lower than 7.The aqueous cation \(H_3O^+\), an oxonium ion type created by protonating water, is known as hydronium in common usage. As an Arrhenius acid dissolves in water, the surrounding water molecules receive a proton from the Arrhenius acid molecules, which is known as a positive hydrogen ion (H+). This is why it is frequently referred to as the positive ion present (\(H_2O\)).
learn more about Acids Refer:brainly.com/question/29796621
#SPJ1
tritearin có công thức phân tử là
Answers
Answer:
C57H110O6
Explanation:
How many grams of KOH are needed to make 0.500 L of a 0.10 M solution
Answers
Answer:
2.8 g KOH
Explanation:
We can find the amount of moles we need by multiplying 0.500 L and the molarity given, 0.10 M (molarity is moles of solute divided by liters of solution). The liters cancel out, leaving us with moles; we can then take the moles and convert it to grams of KOH by using the molar mass of KOH.
0.500 L x 0.10 mol/1 L x 56.106 g /1 mol = 2.8 g KOH (two significant figures)
What is the length of a rectangle with width 10 in. and area 45 in.2
A trinnalo hac height aft and area 32 ft2 What is the length of its b-
Answers
Possible answer could be 900 or 450.
ANSWER ASAP Which part of the picture shows evidence of matter scattering light waves in
many different directions?
O A. The color of the tree appears black against the sky.
O B. The image of the moon in the sky is incomplete.
OC. The image of the moon on the water's surface is distorted.
OD. The colors on the water's surface match the colors in the sky.
Answers
Answer:
The evidence of matter scattering light wave in many different directions is;
D. The colors on the water's surface match the colors in the sky
Explanation:
Light scattering is used to describe the sending of a given beam of light in many directions (by tiny particles) when the light passes through a medium, due to the disruption of the bath of the light ray as it bumps into the tiny particles
Water has the nature of absorbing the red light. The light that enters the water is scattered by the tiny particles in the water such that the blue light which is the spectrum of light water allows to pass through is reflected along with the reflection of the sky and the colors of the water surface match the colors of the sky.
Answer:
C.
Explanation:
I took the quiz
The following differential equation describes a chemical reaction,
dx
dy
=e
−y
(2x+1) where y is the amount of chemical product and x is the length across the reactor. i. Find the particular solution for y, given that y=0 at the edge of the reactor where x=0. [2 marks] ii. Use the particular solution in part i. to find the amount of chemical product, y, at a distance of x=1.
Answers
The amount of chemical product, y, at a distance of x = 1 is given by y = -ln(-(1/2) ln|3| + C3), where C3 is a constant.
The given differential equation is dx/dy = e^(-y)(2x+1), where y represents the amount of chemical product and x represents the length across the reactor.
i. To find the particular solution for y, we need to solve the given differential equation. Let's separate the variables and integrate both sides with respect to x and y.
dx/(2x+1) = e^(-y) dy
Integrating both sides, we get:
∫ dx/(2x+1) = ∫ e^(-y) dy
To integrate the left side, we can use the substitution u = 2x+1. This gives us du = 2dx, which implies dx = du/2.
∫ dx/(2x+1) = ∫ (1/u) (du/2)
= (1/2) ∫ du/u
= (1/2) ln|u| + C1, where C1 is the constant of integration.
= (1/2) ln|2x+1| + C1
Integrating the right side:
∫ e^(-y) dy = -e^(-y) + C2, where C2 is the constant of integration.
Now, equating both sides and simplifying:
(1/2) ln|2x+1| + C1 = -e^(-y) + C2
Rearranging the terms:
e^(-y) = -(1/2) ln|2x+1| + C3, where C3 = C2 - C1.
Taking the natural logarithm of both sides:
-y = ln(-(1/2) ln|2x+1| + C3)
y = -ln(-(1/2) ln|2x+1| + C3), where C3 is a constant.
ii. To find the amount of chemical product, y, at a distance of x = 1, we substitute x = 1 into the particular solution obtained in part i.
y = -ln(-(1/2) ln|2(1)+1| + C3)
Simplifying further:
y = -ln(-(1/2) ln|3| + C3)
Learn more about differential equations from the given link:
brainly.com/question/1164377
#SPJ11
can someone help me with this plz??

Answers
Answer:
I dont know at all and that is confusing.
HELPPPP PLEASE THIS IS FOR THE CATALYSTS LAB
Did you title your lab report?
Did you state the purpose of the investigation?
Did you include a brief overview of the investigation?
Materials and Procedure
Did you make a list of materials? Did you include quantities and SI units?
Did you present the steps of the procedure as a numbered list?
Did you note any changes to the original procedure?
Answers
Answer:
the first thing you do is do your experiment then title it. then state the purpose of the experiment. included a summary of the experiment. make a list of the materials you used. present all the steps in order to make the experiment possible. note any changes to the original procedure. this is basically the steps you have to do in order to make your scientific experiment.
You need a 80% alcohol solution. On hand, you have a 90 mL of a 70% alcohol mixture. You also have 95% alcohol mixture. How much of the 95% mixture will you need to add to obtain the desired solution
Answers
To obtain the desired 80% alcohol solution, you will need to add 60 mL of the 95% alcohol mixture to the 70% alcohol mixture.
To obtain a 80% alcohol solution, you need to determine how much of the 95% alcohol mixture to add to the 70% alcohol mixture.
Let's start by calculating the amount of pure alcohol in the 70% alcohol mixture. Since the mixture is 70% alcohol, we can multiply the volume of the mixture (90 mL) by 0.7 to find the amount of pure alcohol it contains. This gives us 90 mL * 0.7 = 63 mL of pure alcohol.
Next, let's calculate the amount of pure alcohol we need in the final solution. Since we want an 80% alcohol solution, we want 80% of the total volume to be pure alcohol. Let's assume the total volume of the final solution is x mL. Therefore, the amount of pure alcohol we need is 80% of x mL, which is 0.8x mL.
Now, we can set up an equation to solve for x. We have 63 mL of pure alcohol from the 70% mixture, and we need to add a certain amount of the 95% alcohol mixture to obtain the desired 80% solution. So, the equation becomes: 63 mL + amount of pure alcohol from the 95% mixture = 0.8x mL.
To find the amount of pure alcohol from the 95% mixture, we'll assume we need y mL of the 95% mixture. Since the 95% mixture is 95% alcohol, the amount of pure alcohol from the 95% mixture is 0.95y mL.
Substituting these values into the equation, we get: 63 mL + 0.95y mL = 0.8x mL.
To solve for x, we need another equation. Since we have two unknowns, x and y, we'll use the fact that the total volume of the final solution is the sum of the volumes of the two mixtures. Therefore, x mL = 90 mL + y mL.
Now, we can substitute this equation back into the previous equation: 63 mL + 0.95y mL = 0.8(90 mL + y mL).
Simplifying the equation, we get: 63 mL + 0.95y mL = 72 mL + 0.8y mL.
Moving the terms around, we have: 0.15y mL = 9 mL.
Solving for y, we find that y = 60 mL.
Learn more about alcohol solution here:-
https://brainly.com/question/32324620
#SPJ11
Iron filings burn when sprinkled over a gas flame to produce iron(III) oxideWhat type or types of reaction is this?
Answers
Iron filings burning when sprinkled over a gas flame to produce iron(III) oxide is an example of a combustion reaction.
.In this case, the iron filings (Fe) react with oxygen from the gas flame to form iron(III) oxide (Fe₂O₃). The reaction can be represented by the following equation:
4Fe + 3O₂ → 2Fe₂O₃
During the combustion process, the iron filings undergo a chemical change as they combine with oxygen to form a new compound, iron(III) oxide. This reaction is exothermic, meaning it releases energy in the form of heat and light. The heat produced causes the iron filings to burn, resulting in the formation of iron(III) oxide.
Iron(III) oxide is a reddish-brown compound commonly known as rust. It has a different chemical composition and physical properties compared to the original iron filings. The reaction between iron and oxygen is highly exothermic, making it a suitable demonstration for the combustion process.
In summary, the burning of iron filings when sprinkled over a gas flame to produce iron(III) oxide is a combustion reaction where iron reacts with oxygen to form a new compound, releasing heat and light in the process.
Learn more about gas flame
brainly.com/question/28447778
#SPJ11
Calculate the speed for a car that went a distance of 12 000 m in 7200s.
Answers
7200
60
=
120
. Then simplify into hours.
120
60
=
2
. Finally divide the total distance(150km) by the time it took to cover that distance to find the kmph(kilometers per hour).
150
2
=
75
The final answer is 75kmph.
A chemistry needs a small amount of potassium to carry out an experiment in the lab. She discovered that there is no potassium available. Which of the following elements would be the best available replacement? A. calcium B. magnesium C. sodium D. bromine
Answers
The element that we can be able to use for the experiment in place of potassium is sodium.
What is the best replacement for the potassium?We know that the elements that can be found in the same group does react in the same way. Now we know that we have to look about among the options so that we would be able to know element that is in the same group as potassium.
Given that both sodium and potassium are members of group 1, we have to look out for the element that element thus we have to select sodium.
Learn more about group of elements:https://brainly.com/question/5460947
#SPJ1
what is the vsepr geometry is most likely for the carbon atom in this transition state?
Answers
The VSEPR geometry for the carbon atom in the transition state would depend on the specific molecule and reaction being considered.
VSEPR, or Valence Shell Electron Pair Repulsion, the theory is used to predict the shape of molecules based on the distribution of electron pairs around the central atom. In a transition state, the molecule is in a high-energy, intermediate stage of a chemical reaction, and its shape may be different from the starting materials or final products.
The VSEPR geometry of the carbon atom in the transition state would depend on the specifics of the molecule and reaction, including the number of electron pairs around the carbon atom and the types of atoms bonded to it. To determine the VSEPR geometry for a specific transition state, it is necessary to have detailed information about the molecule and reaction in question.
Learn more about VSEPR geometry:
https://brainly.com/question/14225705
#SPJ4